Neopentane

| |||
| |||
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
2,2-Dimethylpropane[2] | |||
| Other names
Neopentane
Tetramethylmethane[1] | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| 1730722 | |||
| ChEBI | |||
| ChemSpider | |||
| ECHA InfoCard | 100.006.677 | ||
| EC Number |
| ||
| 1850 | |||
| MeSH | neopentane | ||
PubChem CID
|
|||
| UNII | |||
| UN number | 2044 | ||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C5H12 | |||
| Molar mass | 72.151 g·mol−1 | ||
| Appearance | Colorless gas | ||
| Odor | Odorless | ||
| Density | 3.255 kg/m3 (gas, 9.5 °C) 601.172 kg/m3 (liquid, 9.5 °C) | ||
| Melting point | −16.5 °C (2.3 °F; 256.6 K) | ||
| Boiling point | 9.5 °C (49.1 °F; 282.6 K) | ||
| Vapor pressure | 146 kPa (at 20 °C)[3] | ||
Henry's law
constant (kH) |
4.7 nmol Pa−1 kg−1 | ||
| Thermochemistry | |||
Heat capacity (C)
|
121.07–120.57 J K−1 mol−1 | ||
Std molar
entropy (S⦵298) |
217 J K−1 mol−1 | ||
Std enthalpy of
formation (ΔfH⦵298) |
−168.5–−167.3 kJ mol−1 | ||
Std enthalpy of
combustion (ΔcH⦵298) |
−3.51506–−3.51314 MJ mol−1 | ||
| Hazards | |||
| GHS labelling: | |||
 
| |||
| Danger | |||
| H220, H411 | |||
| P210, P273, P377, P381, P391, P403, P501 | |||
| NFPA 704 (fire diamond) | |||
| Related compounds | |||
Related alkanes
|
|||
Related compounds
|
|||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
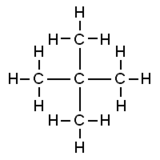
Neopentane, also called 2,2-dimethylpropane, is a double-branched-chain alkane with five carbon atoms. Neopentane is a flammable gas at room temperature and pressure which can condense into a highly volatile liquid on a cold day, in an ice bath, or when compressed to a higher pressure.
Neopentane is the simplest alkane with a quaternary carbon, and has achiral tetrahedral symmetry. It is one of the three structural isomers with the molecular formula C5H12 (pentanes), the other two being n-pentane and isopentane. Out of these three, it is the only one to be a gas at standard conditions; the others are liquids.
It was first synthesized by Russian chemist Mikhail Lvov in 1870.[4]
Nomenclature
[edit]The traditional name neopentane, coined by William Odling in 1876,[5] was still retained in the 1993 IUPAC recommendations,[6][7] but is no longer recommended according to the 2013 recommendations.[2] The preferred IUPAC name is the systematic name 2,2-dimethylpropane, but the substituent numbers are superfluous because it is the only possible “dimethylpropane”.

A neopentyl substituent, often symbolized by "Np", has the structure Me3C–CH2– for instance neopentyl alcohol (Me3CCH2OH or NpOH). As Np also symbolises the element neptunium (atomic number 93) one should use this abbreviation with care.
The obsolete name tetramethylmethane is also used, especially in older sources.[8][9]
Physical properties
[edit]Boiling and melting points
[edit]The boiling point of neopentane is only 9.5 °C, significantly lower than those of isopentane (27.7 °C) and normal pentane (36.0 °C). Therefore, neopentane is a gas at room temperature and atmospheric pressure, while the other two isomers are (barely) liquids.
The melting point of neopentane (−16.6 °C), on the other hand, is 140 degrees higher than that of isopentane (−159.9 °C) and 110 degrees higher than that of n-pentane (−129.8 °C). This anomaly has been attributed to the better solid-state packing assumed to be possible with the tetrahedral neopentane molecule; but this explanation has been challenged on account of it having a lower density than the other two isomers. Moreover, its enthalpy of fusion is lower than the enthalpies of fusion of both n-pentane and isopentane, thus indicating that its high melting point is due to an entropy effect resulting from higher molecular symmetry. Indeed, the entropy of fusion of neopentane is about four times lower than that of n-pentane and isopentane.[10]
1H NMR spectrum
[edit]Because of neopentane's full tetrahedral symmetry, all protons are chemically equivalent, leading to a single NMR chemical shift δ = 0.902 when dissolved in carbon tetrachloride.[11] In this respect, neopentane is similar to its silane analog, tetramethylsilane, whose single chemical shift is zero by convention.
The symmetry of the neopentane molecule can be broken if some hydrogen atoms are replaced by deuterium atoms. In particular, if each methyl group has a different number of substituted atoms (0, 1, 2, and 3), one obtains a chiral molecule. The chirality in this case arises solely by the mass distribution of its nuclei, while the electron distribution is still essentially achiral.[12]
Derivatives
[edit]The alcohol pentaerythritol can be described as the result of replacing one hydrogen in each of the four methyl groups by a hydroxyl (–OH) group.
A linear polymer with alternating neopentane and orthocarbonate groups, which can be described as an ester (pentaerythritol orthocarbonate) with formula [(−CH2)2C(CH2−)2 (−O)2C(O−)2]n, was synthesized in 2002.[13]
References
[edit]- ^ Aston, J.G.; Messerly, G.H., Heat Capacities and Entropies of Organic Compounds II. Thermal and Vapor Pressure Data for Tetramethylmethane from 13.22K to the Boiling Point. The Entropy from its Raman Spectrum, J. Am. Chem. Soc., 1936, 58, 2354.
- ^ a b "Front Matter". Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. p. 652. doi:10.1039/9781849733069-FP001. ISBN 978-0-85404-182-4.
- ^ "Neopentane | C5H12 - PubChem".
- ^ Zeitschrift für Chemie (in German). Quandt & Händel. 1870.
- ^ Philosophical Magazine. Taylor & Francis. 1876.
- ^ Table 19(a) Acyclic and monocyclic hydrocarbons. Parent hydrocarbons
- ^ Panico, R. & Powell, W. H., eds. (1994). A Guide to IUPAC Nomenclature of Organic Compounds 1993. Oxford: Blackwell Science. ISBN 978-0-632-03488-8.
- ^ Whitmore, Frank C.; Fleming, Geo. H. (1934-09-01). "Preparation of Tetramethylmethane (Neopentane) and Determination of its Physical Constants1". Journal of the American Chemical Society. 55 (9): 3803–3806. doi:10.1021/ja01336a058. ISSN 0002-7863.
- ^ LaCoste, Lucien J. B. (1934-10-15). "The Rotational Wave Equation of Tetramethylmethane for Zero Potential and a Generalization". Physical Review. 46 (8): 718–724. Bibcode:1934PhRv...46..718L. doi:10.1103/PhysRev.46.718.
- ^ Wei, James (1999). "Molecular Symmetry, Rotational Entropy, and Elevated Melting Points". Ind. Eng. Chem. Res. 38 (12): 5019–5027. doi:10.1021/ie990588m.
- ^ Spectral Database for Organic Compounds, Proton NMR spectrum of neopentane, accessed 4 Jun 2018.
- ^ Haesler, Jacques; Schindelholz, Ivan; Riguet, Emmanuel; Bochet, Christian G.; Hug, Werner (2007). "Absolute configuration of chirally deuterated neopentane" (PDF). Nature. 446 (7135): 526–529. Bibcode:2007Natur.446..526H. doi:10.1038/nature05653. PMID 17392783. S2CID 4423560.
- ^ Vodak, David T.; Braun, Matthew; Iordanidis, Lykourgos; Plévert, Jacques; Stevens, Michael; Beck, Larry; Spence, John C. H.; O'Keeffe, Michael; Yaghi, Omar M. (2002). "One-Step Synthesis and Structure of an Oligo(spiro-orthocarbonate)". Journal of the American Chemical Society. 124 (18): 4942–4943. doi:10.1021/ja017683i. PMID 11982342.
External links
[edit]- Linstrom, Peter J.; Mallard, William G. (eds.); NIST Chemistry WebBook, NIST Standard Reference Database Number 69, National Institute of Standards and Technology, Gaithersburg (MD)
- IUPAC Nomenclature of Organic Chemistry (online version of the "Blue Book")