Aluminium nitride

| |
| |
| Names | |
|---|---|
| Other names
AlN
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.041.931 |
| EC Number |
|
| 13611 | |
PubChem CID
|
|
| RTECS number |
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| AlN | |
| Molar mass | 40.989 g/mol[1] |
| Appearance | white to pale-yellow solid |
| Density | 3.255 g/cm3[1] |
| Melting point | 2,500 °C (4,530 °F; 2,770 K)[6] |
| hydrolyses (powder), insoluble (monocrystalline) | |
| Solubility | insoluble, subject of hydrolysis in water solutions of bases and acids [2] |
| Band gap | 6.015 eV[3][4] (direct) |
| Electron mobility | ~300 cm2/(V·s) |
| Thermal conductivity | 321 W/(m·K)[5] |
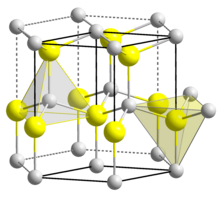
| Structure[7] | |
| Wurtzite | |
| C6v4-P63mc, No. 186, hP4 | |
a = 0.31117 nm, c = 0.49788 nm
| |
Formula units (Z)
|
2 |
| Tetrahedral | |
| Thermochemistry[8] | |
Heat capacity (C)
|
30.1 J/(mol·K) |
Std molar
entropy (S⦵298) |
20.2 J/(mol·K) |
Std enthalpy of
formation (ΔfH⦵298) |
−318.0 kJ/mol |
Gibbs free energy (ΔfG⦵)
|
−287.0 kJ/mol |
| Hazards | |
| GHS labelling: | |
  
| |
| Warning | |
| H315, H319, H335, H373, H411 | |
| P260, P261, P264, P271, P280, P301+P330+P331, P302+P352, P303+P361+P353, P304+P340, P305+P351+P338, P310, P312, P321, P332+P313, P337+P313, P362, P363, P403+P233, P405, P501 | |
| NFPA 704 (fire diamond) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Aluminium nitride (AlN) is a solid nitride of aluminium. It has a high thermal conductivity of up to 321 W/(m·K)[5] and is an electrical insulator. Its wurtzite phase (w-AlN) has a band gap of ~6 eV at room temperature and has a potential application in optoelectronics operating at deep ultraviolet frequencies.
History and physical properties
[edit]AlN was first synthesized in 1862 by F. Briegleb and A. Geuther.[9][10]
AlN, in the pure (undoped) state has an electrical conductivity of 10−11–10−13 Ω−1⋅cm−1, rising to 10−5–10−6 Ω−1⋅cm−1 when doped.[11] Electrical breakdown occurs at a field of 1.2–1.8×105 V/mm (dielectric strength).[11]
The material exists primarily in the hexagonal wurtzite crystal structure, but also has a metastable cubic zincblende phase, which is synthesized primarily in the form of thin films. It is predicted that the cubic phase of AlN (zb-AlN) can exhibit superconductivity at high pressures.[12] In AlN wurtzite crystal structure, Al and N alternate along the c-axis, and each bond is tetrahedrally coordinated with four atoms per unit cell.
One of the unique intrinsic properties of wurtzite AlN is its spontaneous polarization. The origin of spontaneous polarization is the strong ionic character of chemical bonds in wurtzite AlN due to the large difference in electronegativity between aluminium and nitrogen atoms. Furthermore, the non-centrosymmetric wurtzite crystal structure gives rise to a net polarization along the c-axis. Compared with other III-nitride materials, AlN has a larger spontaneous polarization due to the higher nonideality of its crystal structure (Psp: AlN 0.081 C/m2 > InN 0.032 C/m2 > GaN 0.029 C/m2).[13] Moreover, the piezoelectric nature of AlN gives rise to internal piezoelectric polarization charges under strain. These polarization effects can be utilized to induce a high density of free carriers at III-nitride semiconductor heterostructure interfaces completely dispensing with the need of intentional doping. Owing to the broken inversion symmetry along the polar direction, AlN thin film can be grown on either metal-polar or nitrogen-polar faces. Their bulk and surface properties depend significantly on this choice. The polarization effect is currently under investigation for both polarities.
Critical spontaneous and piezoelectric polarization constants for AlN are listed in the table below:[13][14]
| e31
(C/m2) |
e33
(C/m2) |
c13
(GPa) |
c33
(GPa) |
a0
(Å) |
c0
(Å) | |
| AlN | -0.60 | 1.46 | 108 | 373 | 3.112 | 4.982 |
AlN has high thermal conductivity, high-quality MOCVD-grown AlN single crystal has an intrinsic thermal conductivity of 321 W/(m·K), consistent with a first-principle calculation.[5] For an electrically insulating ceramic, it is 70–210 W/(m·K) for polycrystalline material, and as high as 285 W/(m·K) for single crystals).[11]
AlN is one of the few materials that have both a wide and direct bandgap (almost twice that of SiC and GaN) and large thermal conductivity.[15] This is due to its small atomic mass, strong interatomic bonds, and simple crystal structure.[16] This property makes AlN attractive for application in high speed and high power communication networks. Many devices handle and manipulate large amounts of energy in small volumes and at high speeds, so due to the electrically insulating nature and high thermal conductivity of AlN, it becomes a potential material for high-power power electronics. Among group III-nitride materials, AlN has higher thermal conductivity compared to gallium nitride (GaN). Therefore, AlN is more advantageous than GaN in terms of heat dissipation in many power and radio frequency electronic devices.
Thermal expansivity is another critical property for high temperature applications. The calculated thermal expansion coefficients of AlN at 300 K are 4.2×10−6 K−1along a-axis and 5.3×10−6 K−1 along c-axis.[17]
Stability and chemical properties
[edit]Aluminium nitride is stable at high temperatures in inert atmospheres and melts at about 2,200 °C (2,470 K; 3,990 °F). In a vacuum, AlN decomposes at ~1,800 °C (2,070 K; 3,270 °F). In the air, surface oxidation occurs above 700 °C (973 K; 1,292 °F), and even at room temperature, surface oxide layers of 5–10 nm thickness have been detected. This oxide layer protects the material up to 1,370 °C (1,640 K; 2,500 °F). Above this temperature bulk oxidation occurs. Aluminium nitride is stable in hydrogen and carbon-dioxide atmospheres up to 980 °C (1,250 K; 1,800 °F).[18]
The material dissolves slowly in mineral acids through grain-boundary attack and in strong alkalies through attack on the aluminium-nitride grains. The material hydrolyzes slowly in water. Aluminium nitride is resistant to attack from most molten salts, including chlorides and cryolite.[19]
Aluminium nitride can be patterned with a Cl2-based reactive ion etch.[20][21]
Manufacture
[edit]AlN is synthesized by the carbothermal reduction of aluminium oxide in the presence of gaseous nitrogen or ammonia or by direct nitridation of aluminium.[22] The use of sintering aids, such as Y2O3 or CaO, and hot pressing is required to produce a dense technical-grade material.[citation needed]
Applications
[edit]Epitaxially grown thin film crystalline aluminium nitride is used for surface acoustic wave sensors (SAWs) deposited on silicon wafers because of AlN's piezoelectric properties. Recent advancements in material science have permitted the deposition of piezoelectric AlN films on polymeric substrates, thus enabling the development of flexible SAW devices.[23] One application is an RF filter, widely used in mobile phones,[24] which is called a thin-film bulk acoustic resonator (FBAR). This is a MEMS device that uses aluminium nitride sandwiched between two metal layers.[25]
AlN is also used to build piezoelectric micromachined ultrasonic transducers, which emit and receive ultrasound and which can be used for in-air rangefinding over distances of up to a meter.[26][27]
Metallization methods are available to allow AlN to be used in electronics applications similar to those of alumina and beryllium oxide. AlN nanotubes as inorganic quasi-one-dimensional nanotubes, which are isoelectronic with carbon nanotubes, have been suggested as chemical sensors for toxic gases.[28][29]
Currently there is much research into developing light-emitting diodes to operate in the ultraviolet using gallium nitride based semiconductors and, using the alloy aluminium gallium nitride, wavelengths as short as 250 nm have been achieved. In 2006, an inefficient AlN LED emission at 210 nm was reported.[30]
AlN-based high electron mobility transistors (HEMTs) have attracted a high level of attention due to AlN’s superior properties, such as better thermal management, reduced buffer leakage, and excellent integration for all nitride electronics. AlN buffer layer is a critical building block for AlN-based HEMTs, and it has been grown by using MOCVD or MBE on different substrates. Building on top of AlN buffer, n-channel devices with 2D electron gas (2DEG) and p-channel devices with 2D hole gas (2DHG) have been demonstrated. The combination of high-density 2DEG and 2DHG on the same semiconductor platform makes it a potential candidate for CMOS devices.
Aluminum oxide ceramics facilitate polymerization reactions, enhancing efficiency and consistency in creating plastics and resins.[31] They are also used in microwave applications as a substrate and heat sink.[32] More researchers are examining the production of light-emitting diodes(LEDs) to operate in the ultraviolet region using aluminium gallium nitride(AlGaN) based semiconductors.[33]
Among the applications of AlN are
- opto-electronics,
- dielectric layers in optical storage media,
- electronic substrates, chip carriers where high thermal conductivity is essential,
- military applications,
- as a crucible to grow crystals of gallium arsenide,
- steel and semiconductor manufacturing.
See also
[edit]- Boron nitride
- Aluminium phosphide
- Aluminium arsenide
- Aluminium antimonide
- Gallium nitride
- Indium nitride
- Aluminium oxynitride
- Titanium aluminium nitride, TiAlN or AlTiN
References
[edit]- ^ a b Haynes, p. 4.45.
- ^ Fukumoto, S.; Hookabe, T.; Tsubakino, H. (2010). "Hydrolysis behavior of aluminum nitride in various solutions". J. Mat. Science. 35 (11): 2743–2748. doi:10.1023/A:1004718329003. S2CID 91552821.
- ^ Haynes, p. 12.85.
- ^ Feneberg, M.; Leute, R. A. R.; Neuschl, B.; Thonke, K.; Bickermann, M. (2010). Phys. Rev. B. 82 (7): 075208. Bibcode:2010PhRvB..82g5208F. doi:10.1103/physrevb.82.075208.
{{cite journal}}: CS1 maint: untitled periodical (link) - ^ a b c Cheng, Zhe; Koh, Yee Rui; Mamun, Abdullah; Shi, Jingjing; Bai, Tingyu; Huynh, Kenny; Yates, Luke; Liu, Zeyu; Li, Ruiyang; Lee, Eungkyu; Liao, Michael E.; Wang, Yekan; Yu, Hsuan Ming; Kushimoto, Maki; Luo, Tengfei; Goorsky, Mark S.; Hopkins, Patrick E.; Amano, Hiroshi; Khan, Asif; Graham, Samuel (2020). "Experimental observation of high intrinsic thermal conductivity of AlN". Physical Review Materials. 4 (4): 044602. arXiv:1911.01595. Bibcode:2020PhRvM...4d4602C. doi:10.1103/PhysRevMaterials.4.044602. S2CID 207780348. Retrieved 2020-04-03.
- ^ Haynes, p. 12.80.
- ^ Vandamme, Nobuko S.; Richard, Sarah M.; Winzer, Stephen R. (1989). "Liquid-Phase Sintering of Aluminum Nitride by Europium Oxide Additives". Journal of the American Ceramic Society. 72 (8): 1409–1414. doi:10.1111/j.1151-2916.1989.tb07662.x.
- ^ Haynes, p. 5.4.
- ^ Fesenko I. P.; Prokopiv M. M.; Chasnyk V. I.; et al. (2015). Aluminium nitride based functional materials, prepared from nano/micron-sized powders via hot pressing/pressureless sintering. EPC ALCON. p. 11. ISBN 978-966-8449-53-6.
- ^ Briegleb, F.; Geuther, A. (1862). "Ueber das Stickstoffmagnesium und die Affinitäten des Stickgases zu Metallen". Justus Liebigs Annalen der Chemie. 123 (2): 228–241. doi:10.1002/jlac.18621230212.
- ^ a b c "AlN – Aluminium Nitride". Ioffe Database. Sankt-Peterburg: FTI im. A. F. Ioffe, RAN. Retrieved 2014-01-01.
- ^ Dancy, G. Selva; Sheeba, V. Benaline; Louis, C. Nirmala; Amalraj, A. (2015-09-30). "Superconductivity in Group III-V Semiconductor AlN Under High Pressure". Orbital - the Electronic Journal of Chemistry. 7 (3). Instituto de Quimica - Univ. Federal do Mato Grosso do Sul. doi:10.17807/orbital.v7i3.628. ISSN 1984-6428.
- ^ a b Ambacher, O (1998-10-21). "Growth and applications of Group III-nitrides". Journal of Physics D: Applied Physics. 31 (20): 2653–2710. doi:10.1088/0022-3727/31/20/001. ISSN 0022-3727. S2CID 250782290.
- ^ Ambacher, O.; Foutz, B.; Smart, J.; Shealy, J. R.; Weimann, N. G.; Chu, K.; Murphy, M.; Sierakowski, A. J.; Schaff, W. J.; Eastman, L. F.; Dimitrov, R.; Mitchell, A.; Stutzmann, M. (2000-01-01). "Two dimensional electron gases induced by spontaneous and piezoelectric polarization in undoped and doped AlGaN/GaN heterostructures". Journal of Applied Physics. 87 (1): 334–344. Bibcode:2000JAP....87..334A. doi:10.1063/1.371866. ISSN 0021-8979.
- ^ Hickman, Austin Lee; Chaudhuri, Reet; Bader, Samuel James; Nomoto, Kazuki; Li, Lei; Hwang, James C M; Grace Xing, Huili; Jena, Debdeep (2021-04-01). "Next generation electronics on the ultrawide-bandgap aluminum nitride platform". Semiconductor Science and Technology. 36 (4): 044001. Bibcode:2021SeScT..36d4001H. doi:10.1088/1361-6641/abe5fd. ISSN 0268-1242. S2CID 233936255.
- ^ Xu, Runjie Lily; Muñoz Rojo, Miguel; Islam, S. M.; Sood, Aditya; Vareskic, Bozo; Katre, Ankita; Mingo, Natalio; Goodson, Kenneth E.; Xing, Huili Grace; Jena, Debdeep; Pop, Eric (2019-11-14). "Thermal conductivity of crystalline AlN and the influence of atomic-scale defects". Journal of Applied Physics. 126 (18): 185105. arXiv:1904.00345. Bibcode:2019JAP...126r5105X. doi:10.1063/1.5097172. ISSN 0021-8979. S2CID 90262793.
- ^ Slack, Glen A.; Bartram, S. F. (1975-01-01). "Thermal expansion of some diamondlike crystals". Journal of Applied Physics. 46 (1): 89–98. Bibcode:1975JAP....46...89S. doi:10.1063/1.321373. ISSN 0021-8979.
- ^ Berger, L. I. (1997). Semiconductor Materials. CRC Press. pp. 123–124. ISBN 978-0-8493-8912-2.
- ^ Pradhan, S; Jena, S K; Patnaik, S C; Swain, P K; Majhi, J (2015-02-19). "Wear characteristics of Al-AlN composites produced in-situ by nitrogenation". IOP Conference Series: Materials Science and Engineering. 75 (1): 012034. Bibcode:2015MS&E...75a2034P. doi:10.1088/1757-899X/75/1/012034. ISSN 1757-899X. S2CID 137160554.
- ^ Chih-ming Lin; Ting-ta Yen; Yun-ju Lai; Felmetsger, V. V.; Hopcroft, M. A.; Kuypers, J. H.; Pisano, A. P. (March 2010). "Temperature-compensated aluminum nitride lamb wave resonators". IEEE Transactions on Ultrasonics, Ferroelectrics and Frequency Control. 57 (3): 524–532. doi:10.1109/TUFFC.2010.1443. PMID 20211766. S2CID 20028149.
- ^ Xiong, Chi; Pernice, Wolfram H. P.; Sun, Xiankai; Schuck, Carsten; Fong, King Y.; Tang, Hong X. (2012). "Aluminum nitride as a new material for chip-scale optomechanics and nonlinear optics". New Journal of Physics. 14 (9): 095014. arXiv:1210.0975. Bibcode:2012NJPh...14i5014X. doi:10.1088/1367-2630/14/9/095014. ISSN 1367-2630. S2CID 118571039.
- ^ Yamakawa, Tomohiro; Tatami, Junichi; Wakihara, Toru; Komeya, Katsutoshi; Meguro, Takeshi; MacKenzie, Kenneth J. D.; Takagi, Shinichi; Yokouchi, Masahiro (2005-10-04). "Synthesis of AlN Nanopowder from γ-Al2O3 by Reduction-Nitridation in a Mixture of NH3-C3H8". Journal of the American Ceramic Society. 89 (1): 171–175. doi:10.1111/j.1551-2916.2005.00693.x. ISSN 0002-7820. Retrieved 2023-06-26.
- ^ Lamanna, Leonardo (November 2023). "Recent Progress in Polymeric Flexible Surface Acoustic Wave Devices: Materials, Processing, and Applications". Advanced Materials Technologies. 8 (21). doi:10.1002/admt.202300362. ISSN 2365-709X.
- ^ Tsuruoka, Doug (2014-03-17). "Apple, Samsung Cellphone Filter Orders Lift Avago". Investor's Business Daily.
- ^ "ACPF-7001: Agilent Technologies Announces FBAR Filter for U.S. PCS Band Mobile Phones and Data Cards". wirelessZONE. EN-Genius Network Ltd. 2002-05-27. Retrieved 2008-10-18.
- ^ Metz, Rachel (November 2013). "A Gestural Interface for Smart Watches". MIT Technology Review. Archived from the original on Nov 2, 2013.
- ^ Przybyla, R.; al, et (2014). "3D Ultrasonic Gesture Recognition". International Solid State Circuits Conference. San Francisco. pp. 210–211.
- ^ Ahmadi, A.; Hadipour, N. L.; Kamfiroozi, M.; Bagheri, Z. (2012). "Theoretical study of aluminium nitride nanotubes for chemical sensing of formaldehyde". Sensors and Actuators B: Chemical. 161 (1): 1025–1029. Bibcode:2012SeAcB.161.1025A. doi:10.1016/j.snb.2011.12.001.
- ^ Ahmadi Peyghan, A.; Omidvar, A.; Hadipour, N. L.; Bagheri, Z.; Kamfiroozi, M. (2012). "Can aluminum nitride nanotubes detect the toxic NH3 molecules?". Physica E. 44 (7–8): 1357–1360. Bibcode:2012PhyE...44.1357A. doi:10.1016/j.physe.2012.02.018.
- ^ Taniyasu, Y.; et al. (2006). "An Aluminium Nitride Light-Emitting Diode with a Wavelength of 210 Nanometres". Nature. 441 (7091): 325–328. Bibcode:2006Natur.441..325T. doi:10.1038/nature04760. PMID 16710416. S2CID 4373542.
- ^ Ross, Lisa (Apr 12, 2024). "What is Aluminum Nitride Ceramic?". Advanced Ceramic Materials. Retrieved Nov 2, 2024.
- ^ Ma, Yupu; Wei, Tao (2023). "Embedded Microfluidic Cooling in Aluminum Nitride HTCC Substrate for High-Power Radio Frequency Chip Array". Journal of Thermal Science and Engineering Applications. 15 (10): 101004–101012. doi:10.1115/1.4062400.
- ^ Lang, Jing; Xu, Fujun (2024). "Progress in Performance of AlGaN-Based Ultraviolet Light Emitting Diodes". Advanced Electronic Materials: 2300840. doi:10.1002/aelm.202300840.
Cited sources
[edit]- Haynes, William M., ed. (2016). CRC Handbook of Chemistry and Physics (97th ed.). CRC Press. p. 4.45. ISBN 9781498754293.

