Narasin
Appearance
(Redirected from C43H72O11)
 | |
| Clinical data | |
|---|---|
| Other names | (4S)-4-methyl-salinomycin |
| AHFS/Drugs.com | International Drug Names |
| ATCvet code | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.122.892 |
| Chemical and physical data | |
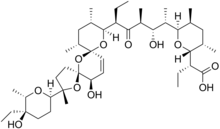
| Formula | C43H72O11 |
| Molar mass | 765.038 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Narasin is a coccidiostat and antibacterial agent.[1][2] It is a derivative of salinomycin with an additional methyl group. Narasin is produced by fermentation of a strain of Streptomyces aureofaciens.[3]
References
[edit]- ^ Gerhold RW, Fuller AL, Lollis L, Parr C, McDougald LR (March 2011). "The efficacy of anticoccidial products against Eimeria spp. in northern bobwhites". Avian Diseases. 55 (1): 59–64. doi:10.1637/9572-101310-Reg.1. PMID 21500637. S2CID 30943649.
- ^ Fitzgerald PR, Mansfield ME (July 1989). "Effects of inoculations with Eimeria zuernii on young calves treated with decoquinate or narasin with or without dexamethasone". American Journal of Veterinary Research. 50 (7): 1056–1059. PMID 2774323.
- ^ Anadón A, Martínez-Larrañaga MR (2014). "Veterinary Drugs Residues: Coccidiostats". Encyclopedia of Food Safety. pp. 63–75. doi:10.1016/B978-0-12-378612-8.00246-8. ISBN 9780123786135.
