Dementia
| Dementia | |
|---|---|
| Other names | Senility,[1] senile dementia |
 | |
| Lithograph of a man diagnosed with dementia in the 1800s | |
| Specialty | Neurology, psychiatry |
| Symptoms | Decreased ability to think and remember, emotional problems, problems with language, decreased motivation, general decline in cognitive abilities[2] |
| Complications | Malnutrition, aspiration pneumonia, inability to perform self-care tasks, personal safety challenges, akinetic mutism[3] |
| Usual onset | Varies, usually gradual[2] |
| Duration | Varies, usually long term[2] |
| Causes | Alzheimer's disease, vascular dementia, Lewy body disease, frontotemporal dementia, and others[2] |
| Risk factors | Lack of education and socialization, family history |
| Diagnostic method | Cognitive testing (mini–mental state examination)[4] |
| Differential diagnosis | Delirium, hypothyroidism[5][6] |
| Prevention | Early education, prevent high blood pressure, prevent obesity, no smoking, social engagement[7] |
| Treatment | Varies, some types can be reversed, but supportive care is given to people suffering from irreversible forms of dementia.[2] |
| Medication | Varies depending on the type, most medications have a small benefit[8] |
| Prognosis | Varies, life expectancy usually shortened |
| Frequency | 55 million (2021)[2] |
| Deaths | 2.4 million (2016)[9] |
Dementia is a syndrome associated with many neurodegenerative diseases, characterized by a general decline in cognitive abilities that affects a person's ability to perform everyday activities. This typically involves problems with memory, thinking, behavior, and motor control.[10] Aside from memory impairment and a disruption in thought patterns, the most common symptoms of dementia include emotional problems, difficulties with language, and decreased motivation.[2] The symptoms may be described as occurring in a continuum over several stages.[11][a] Dementia ultimately has a significant effect on the individual, their caregivers, and their social relationships in general.[2] A diagnosis of dementia requires the observation of a change from a person's usual mental functioning and a greater cognitive decline than might be caused by the normal aging process.[13]
Several diseases and injuries to the brain, such as a stroke, can give rise to dementia. However, the most common cause is Alzheimer's disease, a neurodegenerative disorder.[2] The Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5), has re-described dementia as a mild or major neurocognitive disorder with varying degrees of severity and many causative subtypes. The International Classification of Diseases (ICD-11) also classifies dementia as a neurocognitive disorder (NCD) with many forms or subclasses.[14] Dementia is listed as an acquired brain syndrome, marked by a decline in cognitive function, and is contrasted with neurodevelopmental disorders.[15] It is also described as a spectrum of disorders with causative subtypes of dementia based on a known disorder, such as Parkinson's disease for Parkinson's disease dementia, Huntington's disease for Huntington's disease dementia, vascular disease for vascular dementia, HIV infection causing HIV dementia, frontotemporal lobar degeneration for frontotemporal dementia, Lewy body disease for dementia with Lewy bodies, and prion diseases.[16] Subtypes of neurodegenerative dementias may also be based on the underlying pathology of misfolded proteins, such as synucleinopathies and tauopathies.[16] The coexistence of more than one type of dementia is known as mixed dementia.[15]
Many neurocognitive disorders may be caused by another medical condition or disorder, including brain tumours and subdural hematoma, endocrine disorders such as hypothyroidism and hypoglycemia, nutritional deficiencies including thiamine and niacin, infections, immune disorders, liver or kidney failure, metabolic disorders such as Kufs disease, some leukodystrophies, and neurological disorders such as epilepsy and multiple sclerosis. Some of the neurocognitive deficits may sometimes show improvement with treatment of the causative medical condition.[17]
Diagnosis of dementia is usually based on history of the illness and cognitive testing with imaging. Blood tests may be taken to rule out other possible causes that may be reversible, such as hypothyroidism (an underactive thyroid), and to determine the dementia subtype. One commonly used cognitive test is the mini–mental state examination. Although the greatest risk factor for developing dementia is aging, dementia is not a normal part of the aging process; many people aged 90 and above show no signs of dementia.[18] Several risk factors for dementia, such as smoking and obesity, are preventable by lifestyle changes. Screening the general older population for the disorder is not seen to affect the outcome.[19]
Dementia is currently the seventh leading cause of death worldwide and has 10 million new cases reported every year (approximately one every three seconds).[2] There is no known cure for dementia. Acetylcholinesterase inhibitors such as donepezil are often used and may be beneficial in mild to moderate disorder, but the overall benefit may be minor. There are many measures that can improve the quality of life of a person with dementia and their caregivers. Cognitive and behavioral interventions may be appropriate for treating the associated symptoms of depression.[20]
Signs and symptoms
[edit]The signs and symptoms of dementia are termed as the neuropsychiatric symptoms—also known as the behavioral and psychological symptoms—of dementia.[21][22] The behavioral symptoms can include agitation, restlessness, inappropriate behavior, sexual disinhibition, and verbal or physical aggression.[23] These symptoms may result from impairments in cognitive inhibition.[24] The psychological symptoms can include depression, hallucinations (most often visual),[25] delusions, apathy, and anxiety.[23][26] The most commonly affected areas of brain function include memory, language, attention, problem solving, and visuospatial function affecting perception and orientation. The symptoms progress at a continuous rate over several stages, and they vary across the dementia subtypes.[27][11] Most types of dementia are slowly progressive with some deterioration of the brain well established before signs of the disorder become apparent. There are often other conditions present, such as high blood pressure or diabetes, and there can sometimes be as many as four of these comorbidities.[28]
Signs of dementia include getting lost in a familiar neighborhood, using unusual words to refer to familiar objects, forgetting the name of a close family member or friend, forgetting old memories, and being unable to complete tasks independently.[29] People with developing dementia often fall behind on bill payments; specifically mortgage and credit cards, and a crashing credit score can be an early indicator of the disease.[30][31]
People with dementia are more likely to have problems with incontinence than those of a comparable age without dementia; they are three times more likely to have urinary incontinence and four times more likely to have fecal incontinence.[32][33]
Stages
[edit]The course of dementia is often described in four stages – pre-dementia, early, middle, and late, that show a pattern of progressive cognitive and functional impairment. More detailed descriptions can be arrived at by the use of numeric scales. These scales include the Global Deterioration Scale for Assessment of Primary Degenerative Dementia (GDS or Reisberg Scale), the Functional Assessment Staging Test (FAST), and the Clinical Dementia Rating (CDR).[34] Using the GDS, which more accurately identifies each stage of the disease progression, a more detailed course is described in seven stages – two of which are broken down further into five and six degrees. Stage 7(f) is the final stage.[35][36]
Pre-dementia
[edit]Pre-dementia includes pre-clinical and prodromal stages. The latter stage includes mild cognitive impairment (MCI), delirium-onset, and psychiatric-onset presentations.[37]
Pre-clinical
[edit]Sensory dysfunction is claimed for the pre-clinical stage, which may precede the first clinical signs of dementia by up to ten years.[11] Most notably the sense of smell is lost,[11][38] associated with depression and a loss of appetite leading to poor nutrition.[39] It is suggested that this dysfunction may come about because the olfactory epithelium is exposed to the environment, and the lack of blood–brain barrier protection allows toxic elements to enter and cause damage to the chemosensory networks.[11]
Prodromal
[edit]Pre-dementia states considered as prodromal are mild cognitive impairment (MCI) and mild behavioral impairment (MBI).[40][41][42] Signs and symptoms at the prodromal stage may be subtle, and the early signs often become apparent only in hindsight.[43] Of those diagnosed with MCI, 70% later progress to dementia.[13] In mild cognitive impairment, changes in the person's brain have been happening for a long time, but the symptoms are just beginning to appear. These problems, however, are not severe enough to affect daily function. If and when they do, the diagnosis becomes dementia. The person may have some memory problems and trouble finding words, but they can solve everyday problems and competently handle their life affairs.[44] During this stage, it is ideal to ensure that advance care planning has occurred to protect the person's wishes. Advance directives exist that are specific to sufferers of dementia;[45] these can be particularly helpful in addressing the decisions related to feeding which come with the progression of the illness. Mild cognitive impairment has been relisted in both DSM-5 and ICD-11 as "mild neurocognitive disorders", i.e. milder forms of the major neurocognitive disorder (dementia) subtypes.[46]
Kynurenine is a metabolite of tryptophan that regulates microbiome signaling, immune cell response, and neuronal excitation. A disruption in the kynurenine pathway may be associated with the neuropsychiatric symptoms and cognitive prognosis in mild dementia.[47][48]
Early
[edit]In the early stage of dementia, symptoms become noticeable to other people. In addition, the symptoms begin to interfere with daily activities, and will register a score on a mini–mental state examination (MMSE). MMSE scores are set at 24 to 30 for a normal cognitive rating and lower scores reflect severity of symptoms. The symptoms are dependent on the type of dementia. More complicated chores and tasks around the house or at work become more difficult. The person can usually still take care of themselves but may forget things like taking pills or doing laundry and may need prompting or reminders.[49]
The symptoms of early dementia usually include memory difficulty, but can also include some word-finding problems, and problems with executive functions of planning and organization.[50] Managing finances may prove difficult. Other signs might be getting lost in new places, repeating things, and personality changes.[51]
In some types of dementia, such as dementia with Lewy bodies and frontotemporal dementia, personality changes and difficulty with organization and planning may be the first signs.[52]
Middle
[edit]As dementia progresses, initial symptoms generally worsen. The rate of decline is different for each person. MMSE scores between 6 and 17 signal moderate dementia. For example, people with moderate Alzheimer's dementia lose almost all new information. People with dementia may be severely impaired in solving problems, and their social judgment is often impaired. They cannot usually function outside their own home, and generally should not be left alone. They may be able to do simple chores around the house but not much else, and begin to require assistance for personal care and hygiene beyond simple reminders.[13] A lack of insight into having the condition will become evident.[53][54]
Late
[edit]People with late-stage dementia typically turn increasingly inward and need assistance with most or all of their personal care. People with dementia in the late stages usually need 24-hour supervision to ensure their personal safety, and meeting of basic needs. If left unsupervised, they may wander or fall; may not recognize common dangers such as a hot stove; or may not realize that they need to use the bathroom and become incontinent.[44] They may not want to get out of bed, or may need assistance doing so. Commonly, the person no longer recognizes familiar faces. They may have significant changes in sleeping habits or have trouble sleeping at all.[13]
Changes in eating frequently occur. Cognitive awareness is needed for eating and swallowing and progressive cognitive decline results in eating and swallowing difficulties. This can cause food to be refused, or choked on, and help with feeding will often be required.[55] For ease of feeding, food may be liquidized into a thick purée. They may also struggle to walk, particularly among those with Alzheimer's disease.[56][57][58] In some cases, terminal lucidity, a form of paradoxical lucidity, occurs immediately before death; in this phenomenon, there is an unexpected recovery of mental clarity.[59]
Causes
[edit]Many causes of dementia are neurodegenerative, and protein misfolding is a cardinal feature of these.[60] Other common causes include vascular dementia, dementia with Lewy bodies, frontotemporal dementia, and mixed dementia (commonly Alzheimer's disease and vascular dementia).[2][b][64] Less common causes include normal pressure hydrocephalus, Parkinson's disease dementia, syphilis, HIV, and Creutzfeldt–Jakob disease.[65]
Alzheimer's disease
[edit]
Alzheimer's disease accounts for 60–70% of cases of dementia worldwide. The most common symptoms of Alzheimer's disease are short-term memory loss and word-finding difficulties. Trouble with visuospatial functioning (getting lost often), reasoning, judgment and insight fail. Insight refers to whether or not the person realizes they have memory problems.
The part of the brain most affected by Alzheimer's is the hippocampus. Other parts that show atrophy (shrinking) include the temporal and parietal lobes. Although this pattern of brain shrinkage suggests Alzheimer's, it is variable and a brain scan is insufficient for a diagnosis.
Little is known about the events that occur during and that actually cause Alzheimer's disease. This is due to the fact that, historically, brain tissue from patients with the disease could only be studied after the person's death. Brain scans can now help diagnose and distinguish between different kinds of dementia and show severity. These include magnetic resonance imaging (MRI), computerized tomography (CT), and positron emission tomography (PET). However, it is known that one of the first aspects of Alzheimer's disease is overproduction of amyloid. Extracellular senile plaques (SPs), consisting of beta-amyloid (Aβ) peptides, and intracellular neurofibrillary tangles (NFTs) that are formed by hyperphosphorylated tau proteins, are two well-established pathological hallmarks of AD.[66] Amyloid causes inflammation around the senile plaques of the brain, and too much buildup of this inflammation leads to changes in the brain that cannot be controlled, leading to the symptoms of Alzheimer's.[67]
Several articles have been published on a possible relationship (as an either primary cause or exacerbation of Alzheimer's disease) between general anesthesia and Alzheimer's in specifically the elderly.[68]
Vascular
[edit]Vascular dementia accounts for at least 20% of dementia cases, making it the second most common type.[69] It is caused by disease or injury affecting the blood supply to the brain, typically involving a series of mini-strokes. The symptoms of this dementia depend on where in the brain the strokes occurred and whether the blood vessels affected were large or small.[13] Repeated injury can cause progressive dementia over time, while a single injury located in an area critical for cognition such as the hippocampus, or thalamus, can lead to sudden cognitive decline.[69] Elements of vascular dementia may be present in all other forms of dementia.[70]
Brain scans may show evidence of multiple strokes of different sizes in various locations. People with vascular dementia tend to have risk factors for disease of the blood vessels, such as tobacco use, high blood pressure, atrial fibrillation, high cholesterol, diabetes, or other signs of vascular disease such as a previous heart attack or angina.[71]
Lewy bodies
[edit]The prodromal symptoms of dementia with Lewy bodies (DLB) include mild cognitive impairment, and delirium onset.[72] The symptoms of DLB are more frequent, more severe, and earlier presenting than in the other dementia subtypes.[73] Dementia with Lewy bodies has the primary symptoms of fluctuating cognition, alertness or attention; REM sleep behavior disorder (RBD); one or more of the main features of parkinsonism, not due to medication or stroke; and repeated visual hallucinations.[74] The visual hallucinations in DLB are generally vivid hallucinations of people or animals and they often occur when someone is about to fall asleep or wake up. Other prominent symptoms include problems with planning (executive function) and difficulty with visual-spatial function,[13] and disruption in autonomic bodily functions.[75] Abnormal sleep behaviors may begin before cognitive decline is observed and are a core feature of DLB.[74] RBD is diagnosed either by sleep study recording or, when sleep studies cannot be performed, by medical history and validated questionnaires.[74]
Parkinson's disease
[edit]Parkinson's disease is associated with Lewy body dementia that often progresses to Parkinson's disease dementia following a period of dementia-free Parkinson's disease.[76]
Frontotemporal
[edit]Frontotemporal dementias (FTDs) are characterized by drastic personality changes and language difficulties. In all FTDs, the person has a relatively early social withdrawal and early lack of insight. Memory problems are not a main feature.[13][77] There are six main types of FTD. The first has major symptoms in personality and behavior. This is called behavioral variant FTD (bv-FTD) and is the most common. The hallmark feature of bv-FTD is impulsive behavior, and this can be detected in pre-dementia states.[42] In bv-FTD, the person shows a change in personal hygiene, becomes rigid in their thinking, and rarely acknowledges problems; they are socially withdrawn, and often have a drastic increase in appetite. They may become socially inappropriate. For example, they may make inappropriate sexual comments, or may begin using pornography openly. One of the most common signs is apathy, or not caring about anything. Apathy, however, is a common symptom in many dementias.[13]
Two types of FTD feature aphasia (language problems) as the main symptom. One type is called semantic variant primary progressive aphasia (SV-PPA). The main feature of this is the loss of the meaning of words. It may begin with difficulty naming things. The person eventually may lose the meaning of objects as well. For example, a drawing of a bird, dog, and an airplane in someone with FTD may all appear almost the same.[13] In a classic test for this, a patient is shown a picture of a pyramid and below it a picture of both a palm tree and a pine tree. The person is asked to say which one goes best with the pyramid. In SV-PPA the person cannot answer that question. The other type is called non-fluent agrammatic variant primary progressive aphasia (NFA-PPA). This is mainly a problem with producing speech. They have trouble finding the right words, but mostly they have a difficulty coordinating the muscles they need to speak. Eventually, someone with NFA-PPA only uses one-syllable words or may become totally mute.
A frontotemporal dementia associated with amyotrophic lateral sclerosis (ALS) known as (FTD-ALS) includes the symptoms of FTD (behavior, language and movement problems) co-occurring with amyotrophic lateral sclerosis (loss of motor neurons). Two FTD-related disorders are progressive supranuclear palsy (also classed as a Parkinson-plus syndrome),[78][79] and corticobasal degeneration.[13] These disorders are tau-associated.
Huntington's disease
[edit]Huntington's disease is a neurodegenerative disease caused by mutations in a single gene HTT, that encodes for huntingtin protein. Symptoms include cognitive impairment and this usually declines further into dementia.[80]
The first main symptoms of Huntington's disease often include:
- difficulty concentrating
- memory lapses
- depression - this can include low mood, lack of interest in things, or just abnormal feelings of hopelessness
- stumbling and clumsiness that is out of the ordinary
- mood swings, such as irritability or aggressive behavior to insignificant things[81]
HIV
[edit]HIV-associated dementia results as a late stage from HIV infection, and mostly affects younger people.[82] The essential features of HIV-associated dementia are disabling cognitive impairment accompanied by motor dysfunction, speech problems and behavioral change.[82] Cognitive impairment is characterised by mental slowness, trouble with memory and poor concentration. Motor symptoms include a loss of fine motor control leading to clumsiness, poor balance and tremors. Behavioral changes may include apathy, lethargy and diminished emotional responses and spontaneity. Histopathologically, it is identified by the infiltration of monocytes and macrophages into the central nervous system (CNS), gliosis, pallor of myelin sheaths, abnormalities of dendritic processes and neuronal loss.[83]
Creutzfeldt–Jakob disease
[edit]Creutzfeldt–Jakob disease is a rapidly progressive prion disease that typically causes dementia that worsens over weeks to months. Prions are disease-causing pathogens created from abnormal proteins.[84]
Alcoholism
[edit]Alcohol-related dementia, also called alcohol-related brain damage, occurs as a result of excessive use of alcohol particularly as a substance abuse disorder. Different factors can be involved in this development including thiamine deficiency and age vulnerability.[85][86] A degree of brain damage is seen in more than 70% of those with alcohol use disorder. Brain regions affected are similar to those that are affected by aging, and also by Alzheimer's disease. Regions showing loss of volume include the frontal, temporal, and parietal lobes, as well as the cerebellum, thalamus, and hippocampus.[86] This loss can be more notable, with greater cognitive impairments seen in those aged 65 years and older.[86]
Mixed dementia
[edit]More than one type of dementia, known as mixed dementia, may exist together in about 10% of dementia cases.[2] The most common type of mixed dementia is Alzheimer's disease and vascular dementia.[87] This particular type of mixed dementia's main onsets are a mixture of old age, high blood pressure, and damage to blood vessels in the brain.[15]
Diagnosis of mixed dementia can be difficult, as often only one type will predominate. This makes the treatment of people with mixed dementia uncommon, with many people missing out on potentially helpful treatments. Mixed dementia can mean that symptoms onset earlier, and worsen more quickly since more parts of the brain will be affected.[15]
Other
[edit]Chronic inflammatory conditions that may affect the brain and cognition include Behçet's disease, multiple sclerosis, sarcoidosis, Sjögren's syndrome, lupus, celiac disease, and non-celiac gluten sensitivity.[88][89] These types of dementias can rapidly progress, but usually have a good response to early treatment. This consists of immunomodulators or steroid administration, or in certain cases, the elimination of the causative agent.[89] A 2019 review found no association between celiac disease and dementia overall but a potential association with vascular dementia.[90] A 2018 review found a link between celiac disease or non-celiac gluten sensitivity and cognitive impairment and that celiac disease may be associated with Alzheimer's disease, vascular dementia, and frontotemporal dementia.[91] A strict gluten-free diet started early may protect against dementia associated with gluten-related disorders.[90][91]
Cases of easily reversible dementia include hypothyroidism, vitamin B12 deficiency, Lyme disease, and neurosyphilis. For Lyme disease and neurosyphilis, testing should be done if risk factors are present. Because risk factors are often difficult to determine, testing for neurosyphilis and Lyme disease, as well as other mentioned factors, may be undertaken as a matter of course where dementia is suspected.[13]: 31–32
Many other medical and neurological conditions include dementia only late in the illness. For example, a proportion of patients with Parkinson's disease develop dementia, though widely varying figures are quoted for this proportion.[92] When dementia occurs in Parkinson's disease, the underlying cause may be dementia with Lewy bodies or Alzheimer's disease, or both.[93] Cognitive impairment also occurs in the Parkinson-plus syndromes of progressive supranuclear palsy and corticobasal degeneration (and the same underlying pathology may cause the clinical syndromes of frontotemporal lobar degeneration). Although the acute porphyrias may cause episodes of confusion and psychiatric disturbance, dementia is a rare feature of these rare diseases. Limbic-predominant age-related TDP-43 encephalopathy (LATE) is a type of dementia that primarily affects people in their 80s or 90s and in which TDP-43 protein deposits in the limbic portion of the brain.[94]
Hereditary disorders that can also cause dementia include: some metabolic disorders such as lysosomal storage disorders, leukodystrophies, and spinocerebellar ataxias.
Persistent loneliness may significantly increase the risk of dementia according to a 2024 new study published in Nature Mental Health.[95] Researchers found that loneliness was associated with a 31% higher likelihood of developing any form of dementia, and it also raised the risk of cognitive impairment by 15%.[96]
Diagnosis
[edit]Symptoms are similar across dementia types and it is difficult to diagnose by symptoms alone. Diagnosis may be aided by brain scanning techniques. In many cases, the diagnosis requires a brain biopsy to become final, but this is rarely recommended (though it can be performed at autopsy). In those who are getting older, general screening for cognitive impairment using cognitive testing or early diagnosis of dementia has not been shown to improve outcomes.[19][97] However, screening exams are useful in 65+ persons with memory complaints.[13]
Normally, symptoms must be present for at least six months to support a diagnosis.[98] Cognitive dysfunction of shorter duration is called delirium. Delirium can be easily confused with dementia due to similar symptoms. Delirium is characterized by a sudden onset, fluctuating course, a short duration (often lasting from hours to weeks), and is primarily related to a somatic (or medical) disturbance. In comparison, dementia has typically a long, slow onset (except in the cases of a stroke or trauma), slow decline of mental functioning, as well as a longer trajectory (from months to years).[99]
Some mental illnesses, including depression and psychosis, may produce symptoms that must be differentiated from both delirium and dementia.[100] These are differently diagnosed as pseudodementias, and any dementia evaluation needs to include a depression screening such as the Neuropsychiatric Inventory or the Geriatric Depression Scale.[101][13] Physicians used to think that people with memory complaints had depression and not dementia (because they thought that those with dementia are generally unaware of their memory problems). However, researchers have realized that many older people with memory complaints in fact have mild cognitive impairment the earliest stage of dementia. Depression should always remain high on the list of possibilities, however, for an elderly person with memory trouble. Changes in thinking, hearing and vision are associated with normal ageing and can cause problems when diagnosing dementia due to the similarities.[102] Given the challenging nature of predicting the onset of dementia and making a dementia diagnosis clinical decision making aids underpinned by machine learning and artificial intelligence have the potential to enhance clinical practice.[103]
Cognitive testing
[edit]| Test | Sensitivity | Specificity | Reference |
|---|---|---|---|
| MMSE | 71–92% | 56–96% | [104] |
| 3MS | 83–93% | 85–90% | [105] |
| AMTS | 73–100% | 71–100% | [105] |
Various brief cognitive tests (5–15 minutes) have reasonable reliability to screen for dementia, but may be affected by factors such as age, education and ethnicity.[106] Age and education have a significant influence on the diagnosis of dementia. For example, Individuals with lower education are more likely to be diagnosed with dementia than their educated counterparts.[107] While many tests have been studied,[108][109][110] presently the mini mental state examination (MMSE) is the best studied and most commonly used. The MMSE is a useful tool for helping to diagnose dementia if the results are interpreted along with an assessment of a person's personality, their ability to perform activities of daily living, and their behaviour.[4] Other cognitive tests include the abbreviated mental test score (AMTS), the, "modified mini–mental state examination" (3MS),[111] the Cognitive Abilities Screening Instrument (CASI),[112] the Trail-making test,[113] and the clock drawing test.[34] The MoCA (Montreal Cognitive Assessment) is a reliable screening test and is available online for free in 35 different languages.[13] The MoCA has also been shown somewhat better at detecting mild cognitive impairment than the MMSE.[114][41] People with hearing loss, which commonly occurs alongside dementia, score worse in the MoCA test, which could lead to a false diagnosis of dementia. Researchers have developed an adapted version of the MoCA test, which is accurate and reliable and avoids the need for people to listen and respond to questions.[115][116] The AD-8 – a screening questionnaire used to assess changes in function related to cognitive decline – is potentially useful, but is not diagnostic, is variable, and has risk of bias.[117] An integrated cognitive assessment (CognICA) is a five-minute test that is highly sensitive to the early stages of dementia, and uses an application deliverable to an iPad.[118][119] Previously in use in the UK, in 2021 CognICA was given FDA approval for its commercial use as a medical device.[119]
Another approach to screening for dementia is to ask an informant (relative or other supporter) to fill out a questionnaire about the person's everyday cognitive functioning. Informant questionnaires provide complementary information to brief cognitive tests. Probably the best known questionnaire of this sort is the Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE).[120] Evidence is insufficient to determine how accurate the IQCODE is for diagnosing or predicting dementia.[121] The Alzheimer's Disease Caregiver Questionnaire is another tool. It is about 90% accurate for Alzheimer's when by a caregiver.[13] The General Practitioner Assessment Of Cognition combines both a patient assessment and an informant interview. It was specifically designed for use in the primary care setting.
Clinical neuropsychologists provide diagnostic consultation following administration of a full battery of cognitive testing, often lasting several hours, to determine functional patterns of decline associated with varying types of dementia. Tests of memory, executive function, processing speed, attention and language skills are relevant, as well as tests of emotional and psychological adjustment. These tests assist with ruling out other etiologies and determining relative cognitive decline over time or from estimates of prior cognitive abilities.[122]
Laboratory tests
[edit]Routine blood tests are usually performed to rule out treatable causes. These include tests for vitamin B12, folic acid, thyroid-stimulating hormone (TSH), C-reactive protein, full blood count, electrolytes, calcium, renal function, and liver enzymes. Abnormalities may suggest vitamin deficiency, infection, or other problems that commonly cause confusion or disorientation in the elderly.[123]
Imaging
[edit]A CT scan or MRI scan is commonly performed to possibly find either normal pressure hydrocephalus, a potentially reversible cause of dementia, or connected tumor. The scans can also yield information relevant to other types of dementia, such as infarction (stroke) that would point at a vascular type of dementia. These tests do not pick up diffuse metabolic changes associated with dementia in a person who shows no gross neurological problems (such as paralysis or weakness) on a neurological exam.[124]
The functional neuroimaging modalities of SPECT and PET are more useful in assessing long-standing cognitive dysfunction, since they have shown similar ability to diagnose dementia as a clinical exam and cognitive testing.[125] The ability of SPECT to differentiate vascular dementia from Alzheimer's disease, appears superior to differentiation by clinical exam.[126]
The value of PiB-PET imaging using Pittsburgh compound B (PiB) as a radiotracer has been established in predictive diagnosis, particularly Alzheimer's disease.[127]
Prevention
[edit]Risk factors
[edit]Risk factors for dementia include high blood pressure, high levels of LDL cholesterol, vision loss, hearing loss, smoking, obesity, depression, inactivity, diabetes, lower levels of education and low social contact. Over-indulgence in alcohol, lack of sleep, anemia, traumatic brain injury, and air pollution can also increase the chance of developing dementia.[7][128][129][130] Many of these risk factors, including the lower level of education, smoking, physical inactivity and diabetes, are modifiable.[131] Several of the group are known as vascular risk factors that may be possible to be reduced or eliminated.[132] Managing these risk factors can reduce the risk of dementia in individuals in their late midlife or older age. A reduction in a number of these risk factors can give a positive outcome.[133] The decreased risk achieved by adopting a healthy lifestyle is seen even in those with a high genetic risk.[134]
In addition to the above risk factors, other psychological features, including certain personality traits (high neuroticism, and low conscientiousness), low purpose in life, and high loneliness, are risk factors for Alzheimer's disease and related dementias.[135][136][137] For example, based on the English Longitudinal Study of Ageing (ELSA), research found that loneliness in older people can increase the risk of dementia by one-third. Not having a partner (being single, divorced, or widowed) can double the risk of dementia. However, having two or three closer relationships might reduce the risk by three-fifths.[138][139]
The two most modifiable risk factors for dementia are physical inactivity and lack of cognitive stimulation.[140] Physical activity, in particular aerobic exercise, is associated with a reduction in age-related brain tissue loss, and neurotoxic factors thereby preserving brain volume and neuronal integrity. Cognitive activity strengthens neural plasticity and together they help to support cognitive reserve. The neglect of these risk factors diminishes this reserve.[140]
Sensory impairments of vision and hearing are modifiable risk factors for dementia.[141] These impairments may precede the cognitive symptoms of Alzheimer's disease for example, by many years.[142] Hearing loss may lead to social isolation which negatively affects cognition.[143] Social isolation is also identified as a modifiable risk factor.[142] Age-related hearing loss in midlife is linked to cognitive impairment in late life, and is seen as a risk factor for the development of Alzheimer's disease and dementia. Such hearing loss may be caused by a central auditory processing disorder that makes the understanding of speech against background noise difficult. Age-related hearing loss is characterised by slowed central processing of auditory information.[142][144] Worldwide, mid-life hearing loss may account for around 9% of dementia cases.[145]
Frailty may increase the risk of cognitive decline, and dementia, and the inverse also holds of cognitive impairment increasing the risk of frailty. Prevention of frailty may help to prevent cognitive decline.[142]
There are no medications that can prevent cognitive decline and dementia.[146] However blood pressure lowering medications might decrease the risk of dementia or cognitive problems by around 0.5%.[147]
Economic disadvantage has been shown to have a strong link to higher dementia prevalence,[148] which cannot yet be fully explained by other risk factors.
Dental health
[edit]Limited evidence links poor oral health to cognitive decline. However, failure to perform tooth brushing and gingival inflammation can be used as dementia risk predictors.[149]
Oral bacteria
[edit]The link between Alzheimer's and gum disease is oral bacteria.[150] In the oral cavity, bacterial species include P. gingivalis, F. nucleatum, P. intermedia, and T. forsythia. Six oral treponema spirochetes have been examined in the brains of Alzheimer's patients.[151] Spirochetes are neurotropic in nature, meaning they act to destroy nerve tissue and create inflammation. Inflammatory pathogens are an indicator of Alzheimer's disease and bacteria related to gum disease have been found in the brains of patients with Alzheimer's disease.[151] The bacteria invade nerve tissue in the brain, increasing the permeability of the blood–brain barrier and promoting the onset of Alzheimer's. Individuals with a plethora of tooth plaque risk cognitive decline.[152] Poor oral hygiene can have an adverse effect on speech and nutrition, causing general and cognitive health decline.
Oral viruses
[edit]Herpes simplex virus (HSV) has been found in more than 70% of those aged over 50. HSV persists in the peripheral nervous system and can be triggered by stress, illness or fatigue.[151] High proportions of viral-associated proteins in amyloid plaques or neurofibrillary tangles (NFTs) confirm the involvement of HSV-1 in Alzheimer's disease pathology. NFTs are known as the primary marker of Alzheimer's disease. HSV-1 produces the main components of NFTs.[153]
Diet
[edit]Diet is seen to be a modifiable risk factor for the development of dementia. Thiamine deficiency is identified to increase the risk of Alzheimer's disease in adults.[154] The role of thiamine in brain physiology is unique and essential for the normal cognitive function of older people.[155] Many dietary choices of the elderly population, including the higher intake of gluten-free products, compromise the intake of thiamine as these products are not fortified with thiamine.[156]
The Mediterranean and DASH diets are both associated with less cognitive decline. A different approach has been to incorporate elements of both of these diets into one known as the MIND diet.[157] These diets are generally low in saturated fats while providing a good source of carbohydrates, mainly those that help stabilize blood sugar and insulin levels.[158] Raised blood sugar levels over a long time, can damage nerves and cause memory problems if they are not managed.[159] Nutritional factors associated with the proposed diets for reducing dementia risk include unsaturated fatty acids, vitamin E, vitamin C, flavonoids, vitamin B, and vitamin D.[160][161] A study conducted at the University of Exeter in the United Kingdom seems to have confirmed these findings with fruits, vegetables, whole grains, and healthy fats creating an optimum diet that can help reduce the risk of dementia by roughly 25%.[162]
The MIND diet may be more protective but further studies are needed. The Mediterranean diet seems to be more protective against Alzheimer's than DASH but there are no consistent findings against dementia in general. The role of olive oil needs further study as it may be one of the most important components in reducing the risk of cognitive decline and dementia.[157][163]
In those with celiac disease or non-celiac gluten sensitivity, a strict gluten-free diet may relieve the symptoms given a mild cognitive impairment.[90][91] Once dementia is advanced no evidence suggests that a gluten-free diet is useful.[90]
Omega-3 fatty acid supplements do not appear to benefit or harm people with mild to moderate symptoms.[164] However, there is good evidence that omega-3 incorporation into the diet is of benefit in treating depression, a common symptom,[165] and potentially modifiable risk factor for dementia.[7]
Management
[edit]There are limited options for treating dementia, with most approaches focused on managing or reducing individual symptoms. There are no treatment options available to delay the onset of dementia.[166] Acetylcholinesterase inhibitors are often used early in the disorder course; however, benefit is generally small.[8][167] More than half of people with dementia may experience psychological or behavioral symptoms including agitation, sleep problems, aggression, and/or psychosis. Treatment for these symptoms is aimed at reducing the person's distress and keeping the person safe. Treatments other than medication appear to be better for agitation and aggression.[168] Cognitive and behavioral interventions may be appropriate. Some evidence suggests that education and support for the person with dementia, as well as caregivers and family members, improves outcomes.[169] Palliative care interventions may lead to improvements in comfort in dying, but the evidence is low.[170] Exercise programs are beneficial with respect to activities of daily living, and potentially improve dementia.[171]
The effect of therapies can be evaluated for example by assessing agitation using the Cohen-Mansfield Agitation Inventory (CMAI); by assessing mood and engagement with the Menorah Park Engagement Scale (MPES);[172] and the Observed Emotion Rating Scale (OERS)[173] or by assessing indicators for depression using the Cornell Scale for Depression in Dementia (CSDD)[174] or a simplified version thereof.[175]
Often overlooked in treating and managing dementia is the role of the caregiver and what is known about how they can support multiple interventions. Findings from a 2021 systematic review of the literature found caregivers of people with dementia in nursing homes do not have sufficient tools or clinical guidance for behavioral and psychological symptoms of dementia (BPSD) along with medication use.[176] Simple measures like talking to people about their interests can improve the quality of life for care home residents living with dementia. A programme showed that such simple measures reduced residents' agitation and depression. They also needed fewer GP visits and hospital admissions, which also meant that the programme was cost-saving.[177][178]
Psychological and psychosocial therapies
[edit]Psychological therapies for dementia include some limited evidence for reminiscence therapy (namely, some positive effects in the areas of quality of life, cognition, communication and mood – the first three particularly in care home settings),[179] some benefit for cognitive reframing for caretakers,[180] unclear evidence for validation therapy[181] and tentative evidence for mental exercises, such as cognitive stimulation programs for people with mild to moderate dementia.[182] Offering personally tailored activities may help reduce challenging behavior and may improve quality of life.[183] It is not clear if personally tailored activities have an impact on affect or improve for the quality of life for the caregiver.[183]
Adult daycare centers as well as special care units in nursing homes often provide specialized care for dementia patients. Daycare centers offer supervision, recreation, meals, and limited health care to participants, as well as providing respite for caregivers. In addition, home care can provide one-to-one support and care in the home allowing for more individualized attention that is needed as the disorder progresses. Psychiatric nurses can make a distinctive contribution to people's mental health.[184]
Since dementia impairs normal communication due to changes in receptive and expressive language, as well as the ability to plan and problem solve, agitated behavior is often a form of communication for the person with dementia. Actively searching for a potential cause, such as pain, physical illness, or overstimulation can be helpful in reducing agitation.[185] Additionally, using an "ABC analysis of behavior" can be a useful tool for understanding behavior in people with dementia. It involves looking at the antecedents (A), behavior (B), and consequences (C) associated with an event to help define the problem and prevent further incidents that may arise if the person's needs are misunderstood.[186] The strongest evidence for non-pharmacological therapies for the management of changed behaviors in dementia is for using such approaches.[187] Low quality evidence suggests that regular (at least five sessions of) music therapy may help institutionalized residents. It may reduce depressive symptoms and improve overall behaviors. It may also supply a beneficial effect on emotional well-being and quality of life, as well as reduce anxiety.[188] In 2003, The Alzheimer's Society established 'Singing for the Brain' (SftB) a project based on pilot studies which suggested that the activity encouraged participation and facilitated the learning of new songs. The sessions combine aspects of reminiscence therapy and music.[189] Musical and interpersonal connectedness can underscore the value of the person and improve quality of life.[190]
Some London hospitals found that using color, designs, pictures and lights helped people with dementia adjust to being at the hospital. These adjustments to the layout of the dementia wings at these hospitals helped patients by preventing confusion.[191]
Life story work as part of reminiscence therapy, and video biographies have been found to address the needs of clients and their caregivers in various ways, offering the client the opportunity to leave a legacy and enhance their personhood and also benefitting youth who participate in such work. Such interventions can be more beneficial when undertaken at a relatively early stage of dementia. They may also be problematic in those who have difficulties in processing past experiences[190]
Animal-assisted therapy has been found to be helpful. Drawbacks may be that pets are not always welcomed in a communal space in the care setting. An animal may pose a risk to residents, or may be perceived to be dangerous. Certain animals may also be regarded as "unclean" or "dangerous" by some cultural groups.[190]
Occupational therapy also addresses psychological and psychosocial needs of patients with dementia through improving daily occupational performance and caregivers' competence.[192] When compensatory intervention strategies are added to their daily routine, the level of performance is enhanced and reduces the burden commonly placed on their caregivers.[192] Occupational therapists can also work with other disciplines to create a client centered intervention.[193] To manage cognitive disability, and coping with behavioral and psychological symptoms of dementia, combined occupational and behavioral therapies can support patients with dementia even further.[193]
Cognitive training and rehabilitation
[edit]There is no strong evidence to suggest that cognitive training is beneficial for people with Parkinson's disease, dementia, or mild cognitive impairment.[194] However, a 2023 review found that cognitive rehabilitation may be effective in helping individuals with mild to moderate dementia to manage their daily activities.[195]
Personally tailored activities
[edit]Offering personally tailored activity sessions to people with dementia in long-term care homes may slightly reduce challenging behavior.[196]
Medications
[edit]No medications have been shown to prevent or cure dementia.[197] Medications may be used to treat the behavioral and cognitive symptoms, but have no effect on the underlying disease process.[13][198]
Acetylcholinesterase inhibitors, such as donepezil, may be useful for Alzheimer's disease,[199] Parkinson's disease dementia, DLB, or vascular dementia.[198] The quality of the evidence is poor[200] and the benefit is small.[8] No difference has been shown between the agents in this family.[201] In a minority of people side effects include a slow heart rate and fainting.[202] Rivastigmine is recommended for treating symptoms in Parkinson's disease dementia.[64]
Medications that have anticholinergic effects increase all-cause mortality in people with dementia, although the effect of these medications on cognitive function remains uncertain, according to a systematic review published in 2021.[203]
Before prescribing antipsychotic medication in the elderly, an assessment for an underlying cause of the behavior is needed.[204] Severe and life-threatening reactions occur in almost half of people with DLB,[75][205] and can be fatal after a single dose.[206] People with Lewy body dementias who take neuroleptics are at risk for neuroleptic malignant syndrome, a life-threatening illness.[207] Extreme caution is required in the use of antipsychotic medication in people with DLB because of their sensitivity to these agents.[74] Antipsychotic drugs are used to treat dementia only if non-drug therapies have not worked, and the person's actions threaten themselves or others.[208][209][210][211] Aggressive behavior changes are sometimes the result of other solvable problems, that could make treatment with antipsychotics unnecessary.[208] Because people with dementia can be aggressive, resistant to their treatment, and otherwise disruptive, sometimes antipsychotic drugs are considered as a therapy in response.[208] These drugs have risky adverse effects, including increasing the person's chance of stroke and death.[208] Given these adverse events and small benefit antipsychotics are avoided whenever possible.[187] Generally, stopping antipsychotics for people with dementia does not cause problems, even in those who have been on them a long time.[212]
N-methyl-D-aspartate (NMDA) receptor blockers such as memantine may be of benefit but the evidence is less conclusive than for AChEIs.[199] Due to their differing mechanisms of action memantine and acetylcholinesterase inhibitors can be used in combination however the benefit is slight.[213][214]
An extract of Ginkgo biloba known as EGb 761 has been widely used for treating mild to moderate dementia and other neuropsychiatric disorders.[215] Its use is approved throughout Europe.[216] The World Federation of Biological Psychiatry guidelines lists EGb 761 with the same weight of evidence (level B) given to acetylcholinesterase inhibitors, and memantine. EGb 761 is the only one that showed improvement of symptoms in both AD and vascular dementia. EGb 761 is seen as being able to play an important role either on its own or as an add-on particularly when other therapies prove ineffective.[215] EGb 761 is seen to be neuroprotective; it is a free radical scavenger, improves mitochondrial function, and modulates serotonin and dopamine levels. Many studies of its use in mild to moderate dementia have shown it to significantly improve cognitive function, activities of daily living, neuropsychiatric symptoms, and quality of life.[215][217] However, its use has not been shown to prevent the progression of dementia.[215]
While depression is frequently associated with dementia, the use of antidepressants such as selective serotonin reuptake inhibitors (SSRIs) do not appear to affect outcomes.[218][219] However, the SSRIs sertraline and citalopram have been demonstrated to reduce symptoms of agitation, compared to placebo.[220]
No solid evidence indicates that folate or vitamin B12 improves outcomes in those with cognitive problems.[221] Statins have no benefit in dementia.[222] Medications for other health conditions may need to be managed differently for a person who has a dementia diagnosis. It is unclear whether blood pressure medication and dementia are linked. People may experience an increase in cardiovascular-related events if these medications are withdrawn.[223]
The Medication Appropriateness Tool for Comorbid Health Conditions in Dementia (MATCH-D) criteria can help identify ways that a diagnosis of dementia changes medication management for other health conditions.[224] These criteria were developed because people with dementia live with an average of five other chronic diseases, which are often managed with medications. The systematic review that informed the criteria were published subsequently in 2018 and updated in 2022.[225]
Sleep disturbances
[edit]Over 40% of people with dementia report sleep problems. Approaches to treating these sleep problems include medications and non-pharmacological approaches.[226] The use of medications to alleviate sleep disturbances that people with dementia often experience has not been well researched, even for medications that are commonly prescribed.[227] In 2012 the American Geriatrics Society recommended that benzodiazepines such as diazepam, and non-benzodiazepine hypnotics, be avoided for people with dementia due to the risks of increased cognitive impairment and falls.[228] Benzodiazepines are also known to promote delirium.[229] Additionally, little evidence supports the effectiveness of benzodiazepines in this population.[227][230] No clear evidence shows that melatonin or ramelteon improves sleep for people with dementia due to Alzheimer's,[227] but it is used to treat REM sleep behavior disorder in dementia with Lewy bodies.[75] Limited evidence suggests that a low dose of trazodone may improve sleep, however more research is needed.[227]
Non-pharmacological approaches have been suggested for treating sleep problems for those with dementia, however, there is no strong evidence or firm conclusions on the effectiveness of different types of interventions, especially for those who are living in an institutionalized setting such as a nursing home or long-term care home.[226]
Pain
[edit]As people age, they experience more health problems, and most health problems associated with aging carry a substantial burden of pain; therefore, between 25% and 50% of older adults experience persistent pain. Seniors with dementia experience the same prevalence of conditions likely to cause pain as seniors without dementia.[231] Pain is often overlooked in older adults and, when screened for, is often poorly assessed, especially among those with dementia, since they become incapable of informing others of their pain.[231][232] Beyond the issue of humane care, unrelieved pain has functional implications. Persistent pain can lead to decreased ambulation, depressed mood, sleep disturbances, impaired appetite, and exacerbation of cognitive impairment[232] and pain-related interference with activity is a factor contributing to falls in the elderly.[231][233]
Although persistent pain in people with dementia is difficult to communicate, diagnose, and treat, failure to address persistent pain has profound functional, psychosocial and quality of life implications for this vulnerable population. Health professionals often lack the skills and usually lack the time needed to recognize, accurately assess and adequately monitor pain in people with dementia.[231][234] Family members and friends can make a valuable contribution to the care of a person with dementia by learning to recognize and assess their pain. Educational resources and observational assessment tools are available.[231][235][236]
Eating difficulties
[edit]Persons with dementia may have difficulty eating. Whenever it is available as an option, the recommended response to eating problems is having a caretaker assist them.[208] A secondary option for people who cannot swallow effectively is to consider gastrostomy feeding tube placement as a way to give nutrition. However, in bringing comfort and maintaining functional status while lowering risk of aspiration pneumonia and death, assistance with oral feeding is at least as good as tube feeding.[208][237] Tube-feeding is associated with agitation, increased use of physical and chemical restraints and worsening pressure ulcers. Tube feedings may cause fluid overload, diarrhea, abdominal pain, local complications, less human interaction and may increase the risk of aspiration.[238][239]
Benefits in those with advanced dementia has not been shown.[240] The risks of using tube feeding include agitation, rejection by the person (pulling out the tube, or otherwise physical or chemical immobilization to prevent them from doing this), or developing pressure ulcers.[208] The procedure is directly related to a 1% fatality rate[241] with a 3% major complication rate.[242] The percentage of people at end of life with dementia using feeding tubes in the US has dropped from 12% in 2000 to 6% as of 2014.[243][244]
The immediate and long-term effects of modifying the thickness of fluids for swallowing difficulties in people with dementia are not well known.[245] While thickening fluids may have an immediate positive effect on swallowing and improving oral intake, the long-term impact on the health of the person with dementia should also be considered.[245]
Exercise
[edit]Exercise programs may improve the ability of people with dementia to perform daily activities, but the best type of exercise is still unclear.[246] Getting more exercise can slow the development of cognitive problems such as dementia, proving to reduce the risk of Alzheimer's disease by about 50%. A balance of strength exercise, to help muscles pump blood to the brain, and balance exercises are recommended for aging people. A suggested amount of about 2+1⁄2 hours per week can reduce risks of cognitive decay as well as other health risks like falling.[247]
Assistive technology
[edit]There is a lack of high-quality evidence to determine whether assistive technology effectively supports people with dementia to manage memory issues.[248] Some of the specific things that are used today that helps with dementia today are: clocks, communication aids, electrical appliances the use monitoring, GPS location/ tracking devices, home care robots, in-home cameras, and medication management are just to name a few.[249] Technology has the potential to be a valuable intervention for alleviating loneliness and promoting social connections, supported by available evidence.[250]
Alternative medicine
[edit]Evidence of the therapeutic values of aromatherapy and massage is unclear.[251][252] It is not clear if cannabinoids are harmful or effective for people with dementia.[253]
Palliative care
[edit]Given the progressive and terminal nature of dementia, palliative care can be helpful to patients and their caregivers by helping people with the disorder and their caregivers understand what to expect, deal with loss of physical and mental abilities, support the person's wishes and goals including surrogate decision making, and discuss wishes for or against CPR and life support.[254][255] Because the decline can be rapid, and because most people prefer to allow the person with dementia to make their own decisions, palliative care involvement before the late stages of dementia is recommended.[256][257] Further research is required to determine the appropriate palliative care interventions and how well they help people with advanced dementia.[170]
Person-centered care helps maintain the dignity of people with dementia.[258]
Remotely delivered information for caregivers
[edit]Remotely delivered interventions including support, training and information may reduce the burden for the informal caregiver and improve their depressive symptoms.[259] There is no certain evidence that they improve health-related quality of life.[259]
In several localities in Japan, digital surveillance may be made available to family members, if a dementia patient is prone to wandering and going missing.[260]
Epidemiology
[edit]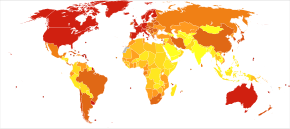
0–4 5–8 9–10 11–13 14–17 | 18–24 25–45 46–114 115–375 376–1266 |

<100 100–120 120–140 140–160 160–180 180–200 | 200–220 220–240 240–260 260–280 280–300 >300 |
The number of cases of dementia worldwide in 2021 was estimated at 55 million, with close to 10 million new cases each year.[2] According to a report by the World Health Organization, "In 2021, Alzheimer’s disease and other forms of dementia ranked as the seventh leading cause of death, killing 1.8 million lives."[261] By 2050, the number of people living with dementia is estimated to be over 150 million globally.[262] Around 7% of people over the age of 65 have dementia, with slightly higher rates (up to 10% of those over 65) in places with relatively high life expectancy.[263] An estimated 58% of people with dementia are living in low and middle income countries.[264][265] The prevalence of dementia differs in different world regions, ranging from 4.7% in Central Europe to 8.7% in North Africa/Middle East; the prevalence in other regions is estimated to be between 5.6 and 7.6%.[264] The number of people living with dementia is estimated to double every 20 years. In 2016 dementia resulted in about 2.4 million deaths,[9] up from 0.8 million in 1990.[266] The genetic and environmental risk factors for dementia disorders vary by ethnicity.[267][268] For instance, Alzheimer's disease among Hispanic/Latino and African American subjects exhibit lower risks associated with gene changes in the apolipoprotein E gene than do non-Hispanic white subjects.[269]
The annual incidence of dementia diagnosis is nearly 10 million worldwide.[170] Almost half of new dementia cases occur in Asia, followed by Europe (25%), the Americas (18%) and Africa (8%). The incidence of dementia increases exponentially with age, doubling with every 6.3-year increase in age.[264] Dementia affects 5% of the population older than 65 and 20–40% of those older than 85.[270] Rates are slightly higher in women than men at ages 65 and greater.[270] The disease trajectory is varied and the median time from diagnosis to death depends strongly on age at diagnosis, from 6.7 years for people diagnosed aged 60–69 to 1.9 years for people diagnosed at 90 or older.[170]
Dementia impacts not only individuals with dementia, but also their carers and the wider society. Among people aged 60 years and over, dementia is ranked the 9th most burdensome condition according to the 2010 Global Burden of Disease (GBD) estimates. The global costs of dementia was around US$818 billion in 2015, a 35.4% increase from US$604 billion in 2010.[264]
A new 2024 study reveals that deaths from dementia in the U.S. have tripled in the past 21 years, rising from around 150,000 in 1999 to over 450,000 in 2020; the likelihood of dying from dementia increased across all demographic groups studied.[271]
Affected ages
[edit]About 3% of people between the ages of 65–74 have dementia, 19% between 75 and 84, and nearly half of those over 85 years of age. As more people are living longer, dementia is becoming more common.[272] For people of a specific age, however, it may be becoming less frequent in the developed world, due to a decrease in modifiable risk factors made possible by greater financial and educational resources. It is one of the most common causes of disability among the elderly but can develop before the age of 65 when it is known as early-onset dementia or presenile dementia.[273][274] Less than 1% of those with Alzheimer's have gene mutations that cause a much earlier development of the disease, around the age of 45, known as early-onset Alzheimer's disease.[275] More than 95% of people with Alzheimer's disease have the sporadic form (late onset, 80–90 years of age).[275] Worldwide the cost of dementia in 2015 was put at US$818 billion. People with dementia are often physically or chemically restrained to a greater degree than necessary, raising issues of human rights.[2][276] Social stigma is commonly perceived by those with the condition, and also by their caregivers.[97]
History
[edit]This section needs additional citations for verification. (November 2015) |
Until the end of the 19th century, dementia was a much broader clinical concept. It included mental illness and any type of psychosocial incapacity, including reversible conditions.[277] Dementia at this time simply referred to anyone who had lost the ability to reason, and was applied equally to psychosis, "organic" diseases like syphilis that destroy the brain, and to the dementia associated with old age, which was attributed to "hardening of the arteries".

Dementia has been referred to in medical texts since antiquity. One of the earliest known allusions to dementia is attributed to the 7th-century BC Greek philosopher Pythagoras, who divided the human lifespan into six distinct phases: 0–6 (infancy), 7–21 (adolescence), 22–49 (young adulthood), 50–62 (middle age), 63–79 (old age), and 80–death (advanced age). The last two he described as the "senium", a period of mental and physical decay, and that the final phase was when "the scene of mortal existence closes after a great length of time that very fortunately, few of the human species arrive at, where the mind is reduced to the imbecility of the first epoch of infancy".[278] In 550 BC, the Athenian statesman and poet Solon argued that the terms of a man's will might be invalidated if he exhibited loss of judgement due to advanced age. Chinese medical texts made allusions to the condition as well, and the characters for "dementia" translate literally to "foolish old person".[279]
Athenian philosophers Aristotle and Plato discussed the mental decline that can come with old age and predicted that this affects everyone who becomes old and nothing can be done to stop this decline from taking place. Plato specifically talked about how the elderly should not be in positions that require responsibility because, "There is not much acumen of the mind that once carried them in their youth, those characteristics one would call judgement, imagination, power of reasoning, and memory. They see them gradually blunted by deterioration and can hardly fulfill their function."[280]
For comparison, the Roman statesman Cicero held a view much more in line with modern-day medical wisdom that loss of mental function was not inevitable in the elderly and "affected only those old men who were weak-willed". He spoke of how those who remained mentally active and eager to learn new things could stave off dementia. However, Cicero's views on aging, although progressive, were largely ignored in a world that would be dominated for centuries by Aristotle's medical writings. Physicians during the Roman Empire, such as Galen and Celsus, simply repeated the beliefs of Aristotle while adding few new contributions to medical knowledge.
Byzantine physicians sometimes wrote of dementia. It is recorded that at least seven emperors whose lifespans exceeded 70 years displayed signs of cognitive decline. In Constantinople, special hospitals housed those diagnosed with dementia or insanity, but these did not apply to the emperors, who were above the law and whose health conditions could not be publicly acknowledged.
Otherwise, little is recorded about dementia in Western medical texts for nearly 1700 years. One of the few references was the 13th-century friar Roger Bacon, who viewed old age as divine punishment for original sin. Although he repeated existing Aristotelian beliefs that dementia was inevitable, he did make the progressive assertion that the brain was the center of memory and thought rather than the heart.
Poets, playwrights, and other writers made frequent allusions to the loss of mental function in old age. William Shakespeare notably mentions it in plays such as Hamlet and King Lear.
During the 19th century, doctors generally came to believe that elderly dementia was the result of cerebral atherosclerosis, although opinions fluctuated between the idea that it was due to blockage of the major arteries supplying the brain or small strokes within the vessels of the cerebral cortex.
In 1907, Bavarian psychiatrist Alois Alzheimer was the first to identify and describe the characteristics of progressive dementia in the brain of 51-year-old Auguste Deter.[281] Deter had begun to behave uncharacteristically, including accusing her husband of adultery, neglecting household chores, exhibiting difficulties writing and engaging in conversations, heightened insomnia, and loss of directional sense.[282] At one point, Deter was reported to have "dragged a bed sheet outside, wandered around wildly, and cried for hours at midnight."[282] Alzheimer began treating Deter when she entered a Frankfurt mental hospital on November 25, 1901.[282] During her ongoing treatment, Deter and her husband struggled to afford the cost of the medical care, and Alzheimer agreed to continue her treatment in exchange for Deter's medical records and donation of her brain upon death.[282] Deter died on April 8, 1906, after succumbing to sepsis and pneumonia.[282] Alzheimer conducted the brain biopsy using the Bielschowsky stain method, which was a new development at the time, and he observed senile plaques, neurofibrillary tangles, and atherosclerotic alteration.[281] At the time, the consensus among medical doctors had been that senile plaques were generally found in older patients, and the occurrence of neurofibrillary tangles was an entirely new observation at the time.[282] Alzheimer presented his findings at the 37th psychiatry conference of southwestern Germany in Tübingen on April 11, 1906; however, the information was poorly received by his peers.[282] By 1910, Alois Alzheimer's teacher, Emil Kraepelin, published a book in which he coined the term "Alzheimer's disease" in an attempt to acknowledge the importance of Alzheimer's discovery.[281][282]
By the 1960s, the link between neurodegenerative diseases and age-related cognitive decline had become more established. By the 1970s, the medical community maintained that vascular dementia was rarer than previously thought and Alzheimer's disease caused the vast majority of old age mental impairments. More recently however, it is believed that dementia is often a mixture of conditions.
In 1976, neurologist Robert Katzmann suggested a link between senile dementia and Alzheimer's disease.[283] Katzmann suggested that much of the senile dementia occurring (by definition) after the age of 65, was pathologically identical with Alzheimer's disease occurring in people under age 65 and therefore should not be treated differently.[284] Katzmann thus suggested that Alzheimer's disease, if taken to occur over age 65, is actually common, not rare, and was the fourth- or 5th-leading cause of death, even though rarely reported on death certificates in 1976.
A helpful finding was that although the incidence of Alzheimer's disease increased with age (from 5–10% of 75-year-olds to as many as 40–50% of 90-year-olds), no threshold was found by which age all persons developed it. This is shown by documented supercentenarians (people living to 110 or more) who experienced no substantial cognitive impairment. Some evidence suggests that dementia is most likely to develop between ages 80 and 84 and individuals who pass that point without being affected have a lower chance of developing it. Women account for a larger percentage of dementia cases than men, although this can be attributed to their longer overall lifespan and greater odds of attaining an age where the condition is likely to occur.[285]
Much like other diseases associated with aging, dementia was comparatively rare before the 20th century, because few people lived past 80. Conversely, syphilitic dementia was widespread in the developed world until it was largely eradicated by the use of penicillin after World War II. With significant increases in life expectancy thereafter, the number of people over 65 started rapidly climbing. While elderly persons constituted an average of 3–5% of the population prior to 1945, by 2010 many countries reached 10–14% and in Germany and Japan, this figure exceeded 20%. Public awareness of Alzheimer's Disease greatly increased in 1994 when former US president Ronald Reagan announced that he had been diagnosed with the condition.
In the 21st century, other types of dementia were differentiated from Alzheimer's disease and vascular dementias (the most common types). This differentiation is on the basis of pathological examination of brain tissues, by symptomatology, and by different patterns of brain metabolic activity in nuclear medical imaging tests such as SPECT and PET scans of the brain. The various forms have differing prognoses and differing epidemiologic risk factors. The main cause for many diseases, including Alzheimer's disease, remains unclear.[286]
Terminology
[edit]Dementia in the elderly was once called senile dementia or senility, and viewed as a normal and somewhat inevitable aspect of aging.[287][288]
By 1913–20 the term dementia praecox was introduced to suggest the development of senile-type dementia at a younger age. Eventually the two terms fused, so that until 1952 physicians used the terms dementia praecox (precocious dementia) and schizophrenia interchangeably. Since then, science has determined that dementia and schizophrenia are two different disorders, though they share some similarities.[289] The term precocious dementia for a mental illness suggested that a type of mental illness like schizophrenia (including paranoia and decreased cognitive capacity) could be expected to arrive normally in all persons with greater age (see paraphrenia). After about 1920, the beginning use of dementia for what is now understood as schizophrenia and senile dementia helped limit the word's meaning to "permanent, irreversible mental deterioration". This began the change to the later use of the term. In recent studies, researchers have seen a connection between those diagnosed with schizophrenia and patients who are diagnosed with dementia, finding a positive correlation between the two diseases.[290]
The view that dementia must always be the result of a particular disease process led for a time to the proposed diagnosis of "senile dementia of the Alzheimer's type" (SDAT) in persons over the age of 65, with "Alzheimer's disease" diagnosed in persons younger than 65 who had the same pathology. Eventually, however, it was agreed that the age limit was artificial, and that Alzheimer's disease was the appropriate term for persons with that particular brain pathology, regardless of age.
After 1952, mental illnesses including schizophrenia were removed from the category of organic brain syndromes, and thus (by definition) removed from possible causes of "dementing illnesses" (dementias). At the same, however, the traditional cause of senile dementia – "hardening of the arteries" – now returned as a set of dementias of vascular cause (small strokes). These were now termed multi-infarct dementias or vascular dementias.
Society and culture
[edit]
The societal cost of dementia is high, especially for caregivers.[291] According to a UK-based study, almost two out of three carers of people with dementia feel lonely. Most of the carers in the study were family members or friends.[292][293]
As of 2015[update], the annual cost per Alzheimer's patient in the United States was around $19,144.36. The total costs for the nation is estimated to be about $167.74 billion. By 2030, it is predicted the annual socioeconomic cost will total to about $507 billion, and by 2050 that number is expected to reach $1.89 trillion. This steady increase will be seen not just within the United States but globally. Global estimates for the costs of dementia were $957.56 billion in 2015, but by 2050 the estimated global cost is $9.12 trillion.[294]
Many countries consider the care of people living with dementia a national priority and invest in resources and education to better inform health and social service workers, unpaid caregivers, relatives and members of the wider community. Several countries have authored national plans or strategies.[295][296] These plans recognize that people can live reasonably with dementia for years, as long as the right support and timely access to a diagnosis are available. Former British Prime Minister David Cameron described dementia as a "national crisis", affecting 800,000 people in the United Kingdom.[297] In fact, dementia has become the leading cause of death for women in England.[298]
There, as with all mental disorders, people with dementia could potentially be a danger to themselves or others, they can be detained under the Mental Health Act 1983 for assessment, care and treatment. This is a last resort, and is usually avoided by people with family or friends who can ensure care.
Some hospitals in Britain work to provide enriched and friendlier care. To make the hospital wards calmer and less overwhelming to residents, staff replaced the usual nurses' station with a collection of smaller desks, similar to a reception area. The incorporation of bright lighting helps increase positive mood and allow residents to see more easily.[299]
Driving with dementia can lead to injury or death. Doctors should advise appropriate testing on when to quit driving.[300] The United Kingdom DVLA (Driver & Vehicle Licensing Agency) states that people with dementia who specifically have poor short-term memory, disorientation, or lack of insight or judgment are not allowed to drive, and in these instances the DVLA must be informed so that the driving license can be revoked. They acknowledge that in low-severity cases and those with an early diagnosis, drivers may be permitted to continue driving.
Many support networks are available to people with dementia and their families and caregivers. Charitable organizations aim to raise awareness and campaign for the rights of people living with dementia. Support and guidance are available on assessing testamentary capacity in people with dementia.[301]
In 2015, Atlantic Philanthropies announced a $177 million gift aimed at understanding and reducing dementia. The recipient was Global Brain Health Institute, a program co-led by the University of California, San Francisco and Trinity College Dublin. This donation is the largest non-capital grant Atlantic has ever made, and the biggest philanthropic donation in Irish history.[302]
In October 2020, the Caretaker's last music release, Everywhere at the End of Time, was popularized by TikTok users for its depiction of the stages of dementia.[303] Caregivers were in favor of this phenomenon; Leyland Kirby, the creator of the record, echoed this sentiment, explaining it could cause empathy among a younger public.[304]
On November 2, 2020, Scottish billionaire Sir Tom Hunter donated £1 million to dementia charities, after watching a former music teacher with dementia, Paul Harvey, playing one of his own compositions on the piano in a viral video. The donation was announced to be split between the Alzheimer's Society and Music for Dementia.[305]
Awareness
Celebrities have used their platforms to raise awareness for the different forms of dementia and the need for further support, including former First Lady of California Maria Shriver, Maria Shriver | My Brain™ | Alzheimer’s Association Academy Award Winning actor Samuel L. Jackson, Editor-in-Chief of ELLE Magazine Nina Garcia, professional skateboarder Tony Hawk, and others.[306]
Additional Alzheimer's awareness has been raised through the diagnoses of high-profile persons themselves, including
- Actor Bruce Willis[307]
- Actor Robin Williams[308]
- Activist Rosa Parks[309]
- 40th President of the United States, Ronald Reagan[310]
- Former Mrs. Colorado Springs Joanna Fix Mrs. Colorado Springs uses title, young-onset Alzheimer's diagnosis to spread awareness about dementia
- TV Host Wendy Williams Wendy Williams diagnosed with same form of dementia as Bruce Willis
- Musician Tony Bennett[311]
- Musician Maureen McGovern[312]
- Dancer and pin-up model Rita Hayworth Rita Hayworth's misdiagnosed struggle
Notes
[edit]- ^ Prodromal subtypes of delirium-onset dementia with Lewy bodies have been proposed as of 2020.[12]
- ^ Kosaka (2017) writes: "Dementia with Lewy bodies (DLB) is now well known to be the second most frequent dementia following Alzheimer disease (AD). Of all types of dementia, AD is known to account for about 50%, DLB about 20% and vascular dementia (VD) about 15%. Thus, AD, DLB, and VD are now considered to be the three major dementias."[61] The NINDS (2020) says that Lewy body dementia "is one of the most common causes of dementia, after Alzheimer's disease and vascular disease."[62] Hershey (2019) says, "DLB is the third most common of all the neurodegenerative diseases behind both Alzheimer's disease and Parkinson's disease".[63]
References
[edit]- ^ "Dementia". medlineplus.gov. Retrieved January 20, 2022.
- ^ a b c d e f g h i j k l m n "Dementia". who.int. Retrieved September 26, 2022.
- ^ "Dementia". mayoclinic.org. Mayo Clinic. Retrieved June 5, 2022.
- ^ a b Creavin ST, Wisniewski S, Noel-Storr AH, Trevelyan CM, Hampton T, Rayment D, et al. (January 2016). "Mini-Mental State Examination (MMSE) for the detection of dementia in clinically unevaluated people aged 65 and over in community and primary care populations". The Cochrane Database of Systematic Reviews. 2016 (1): CD011145. doi:10.1002/14651858.CD011145.pub2. hdl:1983/00876aeb-2061-43f5-b7e1-938c666030ab. PMC 8812342. PMID 26760674.
- ^ "Differential diagnosis dementia". NICE. Retrieved January 20, 2022.
- ^ Hales RE (2008). The American Psychiatric Publishing Textbook of Psychiatry. American Psychiatric Pub. p. 311. ISBN 978-1-58562-257-3. Archived from the original on September 8, 2017.
- ^ a b c Livingston G, Huntley J, Sommerlad A, et al. (August 2020). "Dementia prevention, intervention, and care: 2020 report of the Lancet Commission". Lancet. 396 (10248): 413–446. doi:10.1016/S0140-6736(20)30367-6. PMC 7392084. PMID 32738937.
- ^ a b c Commission de la transparence (June 2012). "Drugs for Alzheimer's disease: best avoided. No therapeutic advantage" [Drugs for Alzheimer's disease: best avoided. No therapeutic advantage]. Prescrire International. 21 (128): 150. PMID 22822592.
- ^ a b Nichols E, Szoeke CE, Vollset SE, Abbasi N, Abd-Allah F, Abdela J, et al. (GBD 2016 Dementia Collaborators) (January 2019). "Global, regional, and national burden of Alzheimer's disease and other dementias, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016". The Lancet. Neurology. 18 (1): 88–106. doi:10.1016/S1474-4422(18)30403-4. PMC 6291454. PMID 30497964.
- ^ "Dementia". who.int. Retrieved April 4, 2024.
- ^ a b c d e Bathini P, Brai E, Auber LA (November 2019). "Olfactory dysfunction in the pathophysiological continuum of dementia" (PDF). Ageing Research Reviews. 55: 100956. doi:10.1016/j.arr.2019.100956. PMID 31479764. S2CID 201742825.
- ^ McKeith IG, Ferman TJ, Thomas AJ, et al. (April 2020). "Research criteria for the diagnosis of prodromal dementia with Lewy bodies". Neurology (Review). 94 (17): 743–755. doi:10.1212/WNL.0000000000009323. PMC 7274845. PMID 32241955.
- ^ a b c d e f g h i j k l m n o p Budson A, Solomon P (2011). Memory loss : a practical guide for clinicians. [Edinburgh?]: Elsevier Saunders. ISBN 978-1-4160-3597-8.
- ^ "ICD-11 for Mortality and Morbidity Statistics". icd.who.int. Retrieved January 20, 2022.
- ^ a b c d "What is mixed dementia". Dementia UK. Archived from the original on November 1, 2020. Retrieved December 13, 2020.
- ^ a b Wilson H, Pagano G, Politis M (March 2019). "Dementia spectrum disorders: lessons learnt from decades with PET research". J Neural Transm (Vienna). 126 (3): 233–251. doi:10.1007/s00702-019-01975-4. PMC 6449308. PMID 30762136.
- ^ American Psychiatric Association (2013). Diagnostic and statistical manual of mental disorders : DSM-5 (5th ed.). Washington, DC: American Psychiatric Association. pp. 591–603. ISBN 978-0-89042-554-1.
- ^ "The Dementias: Hope Through Research | National Institute of Neurological Disorders and Stroke". ninds.nih.gov. Retrieved December 9, 2022.
- ^ a b Lin JS, O'Connor E, Rossom RC, Perdue LA, Eckstrom E (November 2013). "Screening for cognitive impairment in older adults: A systematic review for the U.S. Preventive Services Task Force". Annals of Internal Medicine. 159 (9): 601–612. doi:10.7326/0003-4819-159-9-201311050-00730. PMID 24145578.
- ^ Orgeta V, Leung P, Del-Pino-Casado R, Qazi A, Orrell M, Spector AE, Methley AM (April 2022). "Psychological treatments for depression and anxiety in dementia and mild cognitive impairment". The Cochrane Database of Systematic Reviews. 2022 (4): CD009125. doi:10.1002/14651858.CD009125.pub3. PMC 9035877. PMID 35466396.
- ^ Radue R, Walaszek A, Asthana S (2019). "Chapter 24 – Neuropsychiatric symptoms in dementia". Handbook of Clinical Neurology. Vol. 167. pp. 437–454. doi:10.1016/B978-0-12-804766-8.00024-8. ISBN 978-0-12-804766-8. PMID 31753148. S2CID 208230186.
- ^ Kales HC, Gitlin LN, Lyketsos CG (March 2015). "Assessment and management of behavioral and psychological symptoms of dementia". BMJ. 350: h369. doi:10.1136/bmj.h369. PMC 4707529. PMID 25731881.
- ^ a b Şahin Cankurtaran E (December 2014). "Management of Behavioral and Psychological Symptoms of Dementia". Noro Psikiyatri Arsivi. 51 (4): 303–312. doi:10.5152/npa.2014.7405. PMC 5353163. PMID 28360647.
- ^ "Inhibition in Cognition". apa.org. Retrieved February 7, 2021.
- ^ "Hallucinations: MedlinePlus Medical Encyclopedia". medlineplus.gov. Retrieved December 9, 2022.
- ^ Shub D, Kunik ME (April 16, 2009). "Psychiatric Comorbidity in Persons With Dementia: Assessment and Treatment Strategies". Psychiatric Times. 26 (4). Archived from the original on April 27, 2009.
- ^ "Dementia – Signs and Symptoms". American Speech Language D Association.
- ^ "Dementia: comorbidities in patients – data briefing". GOV.UK. Retrieved November 22, 2020.
- ^ "What Is Dementia? | CDC". cdc.gov. December 19, 2019. Retrieved October 3, 2022.
- ^ Gresenz CR, Mitchell JM, Rodriguez B, Turner RS, van der Klaauw W (May 2024). "The Financial Consequences of Undiagnosed Memory Disorders" (PDF). Federal Reserve Bank of New York Staff Reports. Staff Reports (Federal Reserve Bank of New York) (1106). doi:10.59576/sr.1106. Retrieved July 27, 2024.
- ^ Casselman B (May 31, 2024). "Alzheimer's Takes a Financial Toll Long Before Diagnosis, Study Finds". The New York Times. Archived from the original on June 30, 2024. Retrieved July 27, 2024.
Credit scores among people who later develop dementia begin falling sharply long before their disease is formally identified. A year before diagnosis, these people were 17.2 percent more likely to be delinquent on their mortgage payments than before the onset of the disease, and 34.3 percent more likely to be delinquent on their credit card bills.
- ^ "Continence, dementia, and care that preserves dignity". NIHR Evidence. June 21, 2022. doi:10.3310/nihrevidence_51255. S2CID 251785991.
- ^ Grant RL, Drennan VM, Rait G, Petersen I, Iliffe S (August 2013). Prince MJ (ed.). "First diagnosis and management of incontinence in older people with and without dementia in primary care: a cohort study using The Health Improvement Network primary care database". PLOS Medicine. 10 (8): e1001505. doi:10.1371/journal.pmed.1001505. PMC 3754889. PMID 24015113.
- ^ a b Sheehan B (November 2012). "Assessment scales in dementia". Therapeutic Advances in Neurological Disorders. 5 (6): 349–358. doi:10.1177/1756285612455733. PMC 3487532. PMID 23139705.
- ^ "Seven Stages of Dementia | Symptoms, Progression & Durations". Retrieved December 19, 2020.
- ^ "Clinical Stages of Alzheimer's". Fisher Center for Alzheimer's Research Foundation. January 29, 2014. Retrieved December 19, 2020.
- ^ Scharre DW (June 2019). "Preclinical, Prodromal, and Dementia Stages of Alzheimer's Disease". Practical Neurology. Retrieved June 28, 2022.
- ^ Bhatia-Dey N, Heinbockel T (June 2021). "The Olfactory System as Marker of Neurodegeneration in Aging, Neurological and Neuropsychiatric Disorders". Int J Environ Res Public Health. 18 (13): 6976. doi:10.3390/ijerph18136976. PMC 8297221. PMID 34209997.
- ^ Boesveldt S, Parma V (January 2021). "The importance of the olfactory system in human well-being, through nutrition and social behavior". Cell Tissue Res. 383 (1): 559–567. doi:10.1007/s00441-020-03367-7. PMC 7802608. PMID 33433688.
- ^ Sherman C, Liu CS, Herrmann N, Lanctôt KL (February 2018). "Prevalence, neurobiology, and treatments for apathy in prodromal dementia". Int Psychogeriatr. 30 (2): 177–184. doi:10.1017/S1041610217000527. PMID 28416030. S2CID 46788701.
- ^ a b Breton A, Casey D, Arnaoutoglou NA (February 2019). "Cognitive tests for the detection of mild cognitive impairment (MCI), the prodromal stage of dementia: Meta-analysis of diagnostic accuracy studies". International Journal of Geriatric Psychiatry. 34 (2): 233–242. doi:10.1002/gps.5016. PMID 30370616. S2CID 53097138.
- ^ a b Bateman DR, Gill S, Hu S, Foster ED, Ruthirakuhan MT, Sellek AF, Mortby ME, Matušková V, Ng KP, Tarawneh RM, Freund-Levi Y, Kumar S, Gauthier S, Rosenberg PB, Ferreira de Oliveira F, Devanand DP, Ballard C, Ismail Z (2020). "Agitation and impulsivity in mid and late life as possible risk markers for incident dementia". Alzheimer's & Dementia: Translational Research & Clinical Interventions. 6 (1): e12016. doi:10.1002/trc2.12016. PMC 7507499. PMID 32995467.
- ^ Atri A (March 2019). "The Alzheimer's Disease Clinical Spectrum: Diagnosis and Management". Med Clin North Am. 103 (2): 263–293. doi:10.1016/j.mcna.2018.10.009. PMID 30704681.
- ^ a b Hugo J, Ganguli M (August 2014). "Dementia and cognitive impairment: epidemiology, diagnosis, and treatment". Clinics in Geriatric Medicine. 30 (3): 421–442. doi:10.1016/j.cger.2014.04.001. PMC 4104432. PMID 25037289.
- ^ "Advance Directive for Dementia". dementia-directive.org. Retrieved January 12, 2023.
- ^ American Psychiatric Association (2013). Diagnostic and statistical manual of mental disorders : DSM-5 (5th ed.). Washington, DC: American Psychiatric Association. pp. 591–603. ISBN 978-0-89042-554-1.
- ^ Cervenka I, Agudelo LZ, Ruas JL (July 2017). "Kynurenines: Tryptophan's metabolites in exercise, inflammation, and mental health". Science. 357 (6349): eaaf9794. doi:10.1126/science.aaf9794. PMID 28751584.
- ^ Solvang SH, Nordrehaug JE, Aarsland D, et al. (2019). "Kynurenines, Neuropsychiatric Symptoms, and Cognitive Prognosis in Patients with Mild Dementia". Int J Tryptophan Res. 12: 1178646919877883. doi:10.1177/1178646919877883. PMC 6769202. PMID 31632053.
- ^ Jenkins C, Ginesi L, Keenan B (2016). Dementia care at a glance. Chichester, West Sussex: John Wiley & Sons. ISBN 978-1-118-85998-8. OCLC 905089525.
- ^ Rohrer JD, Knight WD, Warren JE, Fox NC, Rossor MN, Warren JD (January 2008). "Word-finding difficulty: a clinical analysis of the progressive aphasias". Brain. 131 (Pt 1): 8–38. doi:10.1093/brain/awm251. PMC 2373641. PMID 17947337.
- ^ Islam M, Mazumder M, Schwabe-Warf D, Stephan Y, Sutin AR, Terracciano A (February 2019). "Personality Changes With Dementia From the Informant Perspective: New Data and Meta-Analysis". Journal of the American Medical Directors Association. 20 (2): 131–137. doi:10.1016/j.jamda.2018.11.004. PMC 6432780. PMID 30630729.
- ^ "Diagnosing Lewy Body Dementia". National Institute on Aging. Retrieved May 10, 2020.
- ^ Wilson RS, Sytsma J, Barnes LL, Boyle PA (September 2016). "Anosognosia in Dementia". Current Neurology and Neuroscience Reports. 16 (9): 77. doi:10.1007/s11910-016-0684-z. PMID 27438597. S2CID 3331009.
- ^ Sunderaraman P, Cosentino S (March 2017). "Integrating the Constructs of Anosognosia and Metacognition: a Review of Recent Findings in Dementia". Current Neurology and Neuroscience Reports. 17 (3): 27. doi:10.1007/s11910-017-0734-1. PMC 5650061. PMID 28283961.
- ^ Payne M, Morley JE (May 1, 2018). "Editorial: Dysphagia, Dementia and Frailty". The Journal of Nutrition, Health & Aging. 22 (5): 562–565. doi:10.1007/s12603-018-1033-5. PMID 29717753. S2CID 13753522.
- ^ Della Sala S, Spinnler H, Venneri A (February 2004). "Walking difficulties in patients with Alzheimer's disease might originate from gait apraxia" (PDF). Journal of Neurology, Neurosurgery, and Psychiatry. 75 (2): 196–201. PMC 1738895. PMID 14742586. Archived (PDF) from the original on October 9, 2022.
- ^ Mc Ardle R, Galna B, Donaghy P, Thomas A, Rochester L (October 2019). "Do Alzheimer's and Lewy body disease have discrete pathological signatures of gait?". Alzheimer's & Dementia. 15 (10): 1367–1377. doi:10.1016/j.jalz.2019.06.4953. PMID 31548122.
- ^ "Mental, physical and speech abilities in later stages of dementia". Alzheimer's Society. June 29, 2022. Retrieved July 30, 2022.
- ^ Mashour GA, Frank L, Batthyany A, Kolanowski AM, Nahm M, Schulman-Green D, et al. (August 2019). "Paradoxical lucidity: A potential paradigm shift for the neurobiology and treatment of severe dementias". Alzheimer's & Dementia. 15 (8): 1107–1114. doi:10.1016/j.jalz.2019.04.002. hdl:2027.42/153062. PMID 31229433.
- ^ Chung CG, Lee H, Lee SB (September 2018). "Mechanisms of protein toxicity in neurodegenerative diseases". Cell Mol Life Sci. 75 (17): 3159–3180. doi:10.1007/s00018-018-2854-4. PMC 6063327. PMID 29947927.
- ^ Kosaka K, ed. (2017). Dementia with Lewy bodies: clinical and biological aspects (1st ed.). Springer: Japan. doi:10.1007/978-4-431-55948-1. ISBN 978-4-431-55948-1. S2CID 45950966.
- ^ "Lewy body dementia: Hope through research". National Institute of Neurological Disorders and Stroke. US National Institutes of Health. January 10, 2020. Retrieved March 18, 2020.
- ^ Hershey LA, Coleman-Jackson R (April 2019). "Pharmacological management of dementia with Lewy dodies". Drugs Aging (Review). 36 (4): 309–319. doi:10.1007/s40266-018-00636-7. PMC 6435621. PMID 30680679.
- ^ a b Arvanitakis Z, Shah RC, Bennett DA (October 2019). "Diagnosis and Management of Dementia: Review". JAMA. 322 (16): 1589–1599. doi:10.1001/jama.2019.4782. PMC 7462122. PMID 31638686.
- ^ Gauthier S (2006). Clinical diagnosis and management of Alzheimer's disease (3rd ed.). Abingdon, Oxon: Informa Healthcare. pp. 53–54. ISBN 978-0-203-93171-4. Archived from the original on May 3, 2016.
- ^ Abyadeh M, Gupta V, Chitranshi N, Gupta V, Wu Y, Saks D, et al. (April 2021). "Mitochondrial dysfunction in Alzheimer's disease – a proteomics perspective". Expert Review of Proteomics. 18 (4): 295–304. doi:10.1080/14789450.2021.1918550. PMID 33874826. S2CID 233310698.
- ^ Wenk GL (2003). "Neuropathologic changes in Alzheimer's disease". The Journal of Clinical Psychiatry. 64 (Suppl 9): 7–10. PMID 12934968.
- ^ Papon MA, Whittington RA, El-Khoury NB, Planel E (2011). "Alzheimer's disease and anesthesia". Frontiers in Neuroscience. 4: 272. doi:10.3389/fnins.2010.00272. PMC 3034231. PMID 21344011.
- ^ a b Iadecola C (November 2013). "The pathobiology of vascular dementia". Neuron. 80 (4): 844–866. doi:10.1016/j.neuron.2013.10.008. PMC 3842016. PMID 24267647.
- ^ Baskys A, Cheng JX (November 2012). "Pharmacological prevention and treatment of vascular dementia: approaches and perspectives". Exp Gerontol. 47 (11): 887–891. doi:10.1016/j.exger.2012.07.002. PMID 22796225. S2CID 1153876.
- ^ "Vascular dementia – Symptoms and causes". Mayo Clinic. Retrieved July 8, 2021.
- ^ McKeith IG, Ferman TJ, Thomas AJ, et al. (April 2020). "Research criteria for the diagnosis of prodromal dementia with Lewy bodies". Neurology. 94 (17): 743–755. doi:10.1212/WNL.0000000000009323. PMC 7274845. PMID 32241955.
- ^ Jurek L, Herrmann M, Bonze M, et al. (March 1, 2018). "Behavioral and psychological symptoms in Lewy body disease: a review". Gériatrie et Psychologie Neuropsychiatrie du Vieillissement. 16 (1): 87–95. doi:10.1684/pnv.2018.0723. PMID 29569570.
- ^ a b c d McKeith IG, Boeve BF, Dickson DW, et al. (July 2017). "Diagnosis and management of dementia with Lewy bodies: Fourth consensus report of the DLB Consortium". Neurology (Review). 89 (1): 88–100. doi:10.1212/WNL.0000000000004058. PMC 5496518. PMID 28592453.
- ^ a b c Taylor JP, McKeith IG, Burn DJ, et al. (February 2020). "New evidence on the management of Lewy body dementia". Lancet Neurol (Review). 19 (2): 157–169. doi:10.1016/S1474-4422(19)30153-X. PMC 7017451. PMID 31519472. Courtesty link available here.
- ^ Gomperts SN (April 2016). "Lewy Body Dementias: Dementia With Lewy Bodies and Parkinson Disease Dementia". Continuum (Minneap Minn) (Review). 22 (2 Dementia): 435–463. doi:10.1212/CON.0000000000000309. PMC 5390937. PMID 27042903.
- ^ Finger EC (April 2016). "Frontotemporal Dementias". Continuum. 22 (2 Dementia): 464–489. doi:10.1212/CON.0000000000000300. PMC 5390934. PMID 27042904.
- ^ "Progressive Supranuclear Palsy Fact Sheet | National Institute of Neurological Disorders and Stroke". ninds.nih.gov. Retrieved January 20, 2021.
- ^ Lopez G, Bayulkem K, Hallett M (October 2016). "Progressive supranuclear palsy (PSP): Richardson syndrome and other PSP variants". Acta Neurol Scand. 134 (4): 242–249. doi:10.1111/ane.12546. PMC 7292631. PMID 27070344.
- ^ Frank S (January 2014). "Treatment of Huntington's disease". Neurotherapeutics. 11 (1): 153–160. doi:10.1007/s13311-013-0244-z. PMC 3899480. PMID 24366610.
- ^ "Huntington's disease – Symptoms". nhs.uk. February 16, 2018. Retrieved June 28, 2022.
- ^ a b "HIV-Associated Dementia – Neurologic Disorders". MSD Manual Professional Edition.
- ^ Gray F, Adle-Biassette H, Chretien F, Lorin de la Grandmaison G, Force G, Keohane C (2001). "Neuropathology and neurodegeneration in human immunodeficiency virus infection. Pathogenesis of HIV-induced lesions of the brain, correlations with HIV-associated disorders and modifications according to treatments". Clinical Neuropathology. 20 (4): 146–155. PMID 11495003.
- ^ Collinge J (July 2005). "Molecular neurology of prion disease". Journal of Neurology, Neurosurgery, and Psychiatry. 76 (7): 906–919. doi:10.1136/jnnp.2004.048660. PMC 1739714. PMID 15965195.
- ^ Ridley NJ, Draper B, Withall A (2013). "Alcohol-related dementia: an update of the evidence". Alzheimers Res Ther. 5 (1): 3. doi:10.1186/alzrt157. PMC 3580328. PMID 23347747.
- ^ a b c Nunes PT, Kipp BT, Reitz NL, Savage LM (2019). "Aging with alcohol-related brain damage: Critical brain circuits associated with cognitive dysfunction". Late Aging Associated Changes in Alcohol Sensitivity, Neurobehavioral Function, and Neuroinflammation. International Review of Neurobiology. Vol. 148. pp. 101–168. doi:10.1016/bs.irn.2019.09.002. ISBN 978-0-12-817530-9. PMC 7372724. PMID 31733663.
- ^ "What is mixed dementia?". Alzheimer's Society. Retrieved December 13, 2020.
- ^ Schofield P (2005). "Dementia associated with toxic causes and autoimmune disease". International Psychogeriatrics (Review). 17 (Suppl 1): S129–S147. doi:10.1017/s1041610205001997. hdl:1959.13/24647. PMID 16240488. S2CID 11864913.
- ^ a b Rosenbloom MH, Smith S, Akdal G, Geschwind MD (September 2009). "Immunologically mediated dementias". Current Neurology and Neuroscience Reports (Review). 9 (5): 359–367. doi:10.1007/s11910-009-0053-2. PMC 2832614. PMID 19664365.
- ^ a b c d Zis P, Hadjivassiliou M (February 26, 2019). "Treatment of Neurological Manifestations of Gluten Sensitivity and Coeliac Disease". Curr Treat Options Neurol (Review). 21 (3): 10. doi:10.1007/s11940-019-0552-7. PMID 30806821.
- ^ a b c Makhlouf S, Messelmani M, Zaouali J, Mrissa R (2018). "Cognitive impairment in celiac disease and non-celiac gluten sensitivity: review of literature on the main cognitive impairments, the imaging and the effect of gluten free diet". Acta Neurol Belg (Review). 118 (1): 21–27. doi:10.1007/s13760-017-0870-z. PMID 29247390. S2CID 3943047.
- ^ Aarsland D, Kurz MW (February 2010). "The epidemiology of dementia associated with Parkinson disease". Journal of the Neurological Sciences (Review). 289 (1–2): 18–22. doi:10.1016/j.jns.2009.08.034. PMID 19733364. S2CID 24541533.
- ^ Galvin JE, Pollack J, Morris JC (November 2006). "Clinical phenotype of Parkinson disease dementia". Neurology. 67 (9): 1605–1611. doi:10.1212/01.wnl.0000242630.52203.8f. PMID 17101891. S2CID 25023606.
- ^ Abbasi J (September 2019). "Debate Sparks Over LATE, a Recently Recognized Dementia". JAMA. 322 (10): 914–916. doi:10.1001/jama.2019.12232. PMID 31433447. S2CID 201118832.
- ^ Mundell E (October 10, 2024). "Loneliness Raises Odds for Dementia by 31%". www.healthday.com. Retrieved October 12, 2024.
- ^ Luchetti M, Aschwanden D, Sesker AA, Zhu X, O'Súilleabháin PS, Stephan Y, Terracciano A, Sutin AR (2024). "A meta-analysis of loneliness and risk of dementia using longitudinal data from >600,000 individuals". Nature Mental Health: 1–12. doi:10.1038/s44220-024-00328-9.
- ^ a b Martin S, Kelly S, Khan A, Cullum S, Dening T, Rait G, Fox C, Katona C, Cosco T, Brayne C, Lafortune L (June 2015). "Attitudes and preferences towards screening for dementia: a systematic review of the literature". BMC Geriatr. 15: 66. doi:10.1186/s12877-015-0064-6. PMC 4469007. PMID 26076729.
- ^ "Dementia definition". MDGuidelines. Reed Group. Archived from the original on June 29, 2009. Retrieved June 4, 2009.
- ^ Caplan JP, Rabinowitz T (November 2010). "An approach to the patient with cognitive impairment: delirium and dementia". The Medical Clinics of North America. 94 (6): 1103–1116, ix. doi:10.1016/j.mcna.2010.08.004. PMID 20951272.
- ^ Gleason OC (March 2003). "Delirium". American Family Physician. 67 (5): 1027–1034. PMID 12643363. Archived from the original on September 29, 2007.
- ^ Lai CK (2014). "The merits and problems of Neuropsychiatric Inventory as an assessment tool in people with dementia and other neurological disorders". Clinical Interventions in Aging. 9: 1051–1061. doi:10.2147/CIA.S63504. PMC 4099101. PMID 25031530.
- ^ Worrall L, Hickson LM (2003). "Implications for theory, practice, and policy". In Worrall LE, Hickson LM (eds.). Communication disability in aging: from prevention to intervention. Clifton Park, NY: Delmar Learning. pp. 297–298. ISBN 978-0-7693-0015-3.
- ^ James C, Ranson JM, Everson R, Llewellyn DJ (December 2021). "Performance of Machine Learning Algorithms for Predicting Progression to Dementia in Memory Clinic Patients". JAMA Network Open. 4 (12): e2136553. doi:10.1001/jamanetworkopen.2021.36553. PMC 8678688. PMID 34913981.
- ^ Boustani M, Peterson B, Hanson L, Harris R, Lohr KN (June 2003). "Screening for dementia in primary care: a summary of the evidence for the U.S. Preventive Services Task Force". Annals of Internal Medicine. 138 (11): 927–937. doi:10.7326/0003-4819-138-11-200306030-00015. PMID 12779304. S2CID 20779164.
- ^ a b Cullen B, O'Neill B, Evans JJ, Coen RF, Lawlor BA (August 2007). "A review of screening tests for cognitive impairment". Journal of Neurology, Neurosurgery, and Psychiatry. 78 (8): 790–799. doi:10.1136/jnnp.2006.095414. PMC 2117747. PMID 17178826.
- ^ Ranson JM, Kuźma E, Hamilton W, Muniz-Terrera G, Langa KM, Llewellyn DJ (April 2019). "Predictors of dementia misclassification when using brief cognitive assessments". Neurology. Clinical Practice. 9 (2): 109–117. doi:10.1212/CPJ.0000000000000566. PMC 6461420. PMID 31041124.
- ^ Contador, I. et al. (2017) "Impact of literacy and years of education on the diagnosis of dementia:A population-based study,” Journal of Clinical and Experimental Neuropsychology, 39(2), pp. 112–119. Available at: https://doi.org/10.1080/13803395.2016.1204992.
- ^ Sager MA, Hermann BP, La Rue A, Woodard JL (October 2006). "Screening for dementia in community-based memory clinics" (PDF). WMJ. 105 (7): 25–29. PMID 17163083. Archived from the original (PDF) on June 26, 2010.
- ^ Fleisher AS, Sowell BB, Taylor C, Gamst AC, Petersen RC, Thal LJ (May 2007). "Clinical predictors of progression to Alzheimer disease in amnestic mild cognitive impairment". Neurology. 68 (19): 1588–1595. doi:10.1212/01.wnl.0000258542.58725.4c. PMID 17287448. S2CID 9129604.
- ^ Karlawish JH, Clark CM (March 2003). "Diagnostic evaluation of elderly patients with mild memory problems". Annals of Internal Medicine. 138 (5): 411–419. doi:10.7326/0003-4819-138-5-200303040-00011. PMID 12614094. S2CID 43798118.
- ^ Teng EL, Chui HC (August 1987). "The Modified Mini-Mental State (3MS) examination". The Journal of Clinical Psychiatry. 48 (8): 314–318. PMID 3611032.
- ^ Teng EL, Hasegawa K, Homma A, Imai Y, Larson E, Graves A, et al. (1994). "The Cognitive Abilities Screening Instrument (CASI): a practical test for cross-cultural epidemiological studies of dementia". International Psychogeriatrics. 6 (1): 45–58, discussion 62. doi:10.1017/S1041610294001602. PMID 8054493. S2CID 25322040.
- ^ Tombaugh TN (March 2004). "Trail Making Test A and B: normative data stratified by age and education". Archives of Clinical Neuropsychology. 19 (2): 203–214. doi:10.1016/S0887-6177(03)00039-8. PMID 15010086.
- ^ Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, et al. (April 2005). "The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment". Journal of the American Geriatrics Society. 53 (4): 695–699. doi:10.1111/j.1532-5415.2005.53221.x. PMID 15817019. S2CID 9014589.
- ^ Dawes P, Reeves D, Yeung WK, Holland F, Charalambous AP, Côté M, et al. (May 2023). "Development and validation of the Montreal cognitive assessment for people with hearing impairment (MoCA-H)". Journal of the American Geriatrics Society. 71 (5): 1485–1494. doi:10.1111/jgs.18241. PMID 36722180. S2CID 256457783.
- ^ "How to identify dementia in people with hearing loss". NIHR Evidence. February 8, 2023. doi:10.3310/nihrevidence_59137. S2CID 260465275.
- ^ Hendry K, Green C, McShane R, Noel-Storr AH, Stott DJ, Anwer S, et al. (March 2019). "AD-8 for detection of dementia across a variety of healthcare settings". The Cochrane Database of Systematic Reviews. 3 (3): CD011121. doi:10.1002/14651858.CD011121.pub2. PMC 6398085. PMID 30828783.
- ^ Bee P. "The five-minute test that can tell if you're on the road to dementia". Retrieved January 1, 2022.
- ^ a b "FDA Clears 5-Minute Test for Early Dementia". Medscape. Retrieved January 1, 2022.
- ^ Jorm AF (September 2004). "The Informant Questionnaire on cognitive decline in the elderly (IQCODE): a review". International Psychogeriatrics. 16 (3): 275–293. doi:10.1017/S1041610204000390. PMID 15559753. S2CID 145256616.
- ^ Burton JK, Stott DJ, McShane R, Noel-Storr AH, Swann-Price RS, Quinn TJ (July 2021). "Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE) for the early detection of dementia across a variety of healthcare settings". The Cochrane Database of Systematic Reviews. 2021 (7): CD011333. doi:10.1002/14651858.CD011333.pub3. PMC 8406787. PMID 34275145.
- ^ Jacova C, Kertesz A, Blair M, Fisk JD, Feldman HH (October 2007). "Neuropsychological testing and assessment for dementia". Alzheimer's & Dementia. 3 (4): 299–317. doi:10.1016/j.jalz.2007.07.011. PMID 19595951. S2CID 40462470.
- ^ Boise L, Camicioli R, Morgan DL, Rose JH, Congleton L (August 1999). "Diagnosing dementia: perspectives of primary care physicians". The Gerontologist. 39 (4): 457–464. doi:10.1093/geront/39.4.457. PMID 10495584.
- ^ Espino DV, Jules-Bradley AC, Johnston CL, Mouton CP (March 1998). "Diagnostic approach to the confused elderly patient". American Family Physician. 57 (6): 1358–1366. PMID 9531917.
- ^ Bonte FJ, Harris TS, Hynan LS, Bigio EH, White CL (July 2006). "Tc-99m HMPAO SPECT in the differential diagnosis of the dementias with histopathologic confirmation". Clinical Nuclear Medicine. 31 (7): 376–378. doi:10.1097/01.rlu.0000222736.81365.63. PMID 16785801. S2CID 39518497.
- ^ Dougall NJ, Bruggink S, Ebmeier KP (2004). "Systematic review of the diagnostic accuracy of 99mTc-HMPAO-SPECT in dementia" (PDF). The American Journal of Geriatric Psychiatry. 12 (6): 554–570. doi:10.1176/appi.ajgp.12.6.554. PMID 15545324. S2CID 12375536. Archived from the original (PDF) on July 13, 2020.
- ^ Angelopoulou E, Paudel YN, Shaikh MF, Piperi C (March 2020). "Flotillin: A Promising Biomarker for Alzheimer's Disease". J Pers Med. 10 (2): 20. doi:10.3390/jpm10020020. PMC 7354424. PMID 32225073.
- ^ Zhang YR, Xu W, Zhang W, et al. (October 2022). "Modifiable risk factors for incident dementia and cognitive impairment: An umbrella review of evidence". J Affect Disord. 314: 160–167. doi:10.1016/j.jad.2022.07.008. PMID 35863541.
- ^ Livingston G, Huntley J, Liu KY, Costafreda SG, Selbæk G, Alladi S, Ames D, Banerjee S, Burns A, Brayne C, Fox NC, Ferri CP, Gitlin LN, Howard R, Kales HC (August 2024). "Dementia prevention, intervention, and care: 2024 report of the Lancet standing Commission". The Lancet. 404 (10452): 572–628. doi:10.1016/S0140-6736(24)01296-0.
- ^ Larson EB, Gitlin L (August 12, 2024). "Dementia risk factors identified in new global report are all preventable – addressing them could reduce dementia rates by 45%". The Conversation. Retrieved October 17, 2024.
- ^ Huntley J, Corbett A, Wesnes K, Brooker H, Stenton R, Hampshire A, Ballard C (February 2018). "Online assessment of risk factors for dementia and cognitive function in healthy adults". International Journal of Geriatric Psychiatry. 33 (2): e286–e293. doi:10.1002/gps.4790. PMID 28960500. S2CID 33822160.
- ^ "vascular risk factors and brain health" (PDF). Archived (PDF) from the original on October 9, 2022. Retrieved January 1, 2021.
- ^ Ding J, Davis-Plourde KL, Sedaghat S, Tully PJ, Wang W, Phillips C, et al. (January 2020). "Antihypertensive medications and risk for incident dementia and Alzheimer's disease: a meta-analysis of individual participant data from prospective cohort studies". The Lancet. Neurology. 19 (1): 61–70. doi:10.1016/S1474-4422(19)30393-X. PMC 7391421. PMID 31706889.
- ^ Lourida I, Hannon E, Littlejohns TJ, Langa KM, Hyppönen E, Kuzma E, Llewellyn DJ (July 2019). "Association of Lifestyle and Genetic Risk With Incidence of Dementia". JAMA. 322 (5): 430–437. doi:10.1001/jama.2019.9879. PMC 6628594. PMID 31302669.
- ^ Aschwanden D, Strickhouser JE, Luchetti M, Stephan Y, Sutin AR, Terracciano A (May 2021). "Is personality associated with dementia risk? A meta-analytic investigation". Ageing Research Reviews. 67: 101269. doi:10.1016/j.arr.2021.101269. PMC 8005464. PMID 33561581.
- ^ Sutin AR, Aschwanden D, Luchetti M, Stephan Y, Terracciano A (2021). "Sense of Purpose in Life Is Associated with Lower Risk of Incident Dementia: A Meta-Analysis". Journal of Alzheimer's Disease. 83 (1): 249–258. doi:10.3233/JAD-210364. PMC 8887819. PMID 34275900.
- ^ Luchetti M, Terracciano A, Aschwanden D, Lee JH, Stephan Y, Sutin AR (July 2020). "Loneliness is associated with risk of cognitive impairment in the Survey of Health, Ageing and Retirement in Europe". International Journal of Geriatric Psychiatry. 35 (7): 794–801. doi:10.1002/gps.5304. PMC 7755119. PMID 32250480.
- ^ "Loneliness, but not social isolation, predicts development of dementia in older people". NIHR Evidence (Plain English summary). May 27, 2020. doi:10.3310/alert_40330. S2CID 241649845.
- ^ Rafnsson SB, Orrell M, d'Orsi E, Hogervorst E, Steptoe A (January 2020). Carr D (ed.). "Loneliness, Social Integration, and Incident Dementia Over 6 Years: Prospective Findings From the English Longitudinal Study of Ageing". The Journals of Gerontology. Series B, Psychological Sciences and Social Sciences. 75 (1): 114–124. doi:10.1093/geronb/gbx087. PMC 6909434. PMID 28658937.
- ^ a b Cheng ST (September 2016). "Cognitive Reserve and the Prevention of Dementia: the Role of Physical and Cognitive Activities". Current Psychiatry Reports. 18 (9): 85. doi:10.1007/s11920-016-0721-2. PMC 4969323. PMID 27481112.
- ^ Dawes P (March 2019). "Hearing interventions to prevent dementia". HNO. 67 (3): 165–171. doi:10.1007/s00106-019-0617-7. PMC 6399173. PMID 30767054.
- ^ a b c d Panza F, Lozupone M, Sardone R, Battista P, Piccininni M, Dibello V, et al. (2019). "Sensorial frailty: age-related hearing loss and the risk of cognitive impairment and dementia in later life". Therapeutic Advances in Chronic Disease. 10: 2040622318811000. doi:10.1177/2040622318811000. PMC 6700845. PMID 31452865.
- ^ Thomson RS, Auduong P, Miller AT, Gurgel RK (April 2017). "Hearing loss as a risk factor for dementia: A systematic review". Laryngoscope Investigative Otolaryngology. 2 (2): 69–79. doi:10.1002/lio2.65. PMC 5527366. PMID 28894825.
- ^ Hubbard HI, Mamo SK, Hopper T (July 2018). "Dementia and Hearing Loss: Interrelationships and Treatment Considerations". Seminars in Speech and Language. 39 (3): 197–210. doi:10.1055/s-0038-1660779. PMID 29933487. S2CID 49383232.
- ^ Ford AH, Hankey GJ, Yeap BB, Golledge J, Flicker L, Almeida OP (June 2018). "Hearing loss and the risk of dementia in later life". Maturitas. 112: 1–11. doi:10.1016/j.maturitas.2018.03.004. PMID 29704910. S2CID 13998812.
- ^ Fink HA, Jutkowitz E, McCarten JR, Hemmy LS, Butler M, Davila H, et al. (January 2018). "Pharmacologic Interventions to Prevent Cognitive Decline, Mild Cognitive Impairment, and Clinical Alzheimer-Type Dementia: A Systematic Review". Annals of Internal Medicine. 168 (1): 39–51. doi:10.7326/M17-1529. PMID 29255847. S2CID 24193907.
- ^ Hughes D, Judge C, Murphy R, Loughlin E, Costello M, Whiteley W, et al. (May 2020). "Association of Blood Pressure Lowering With Incident Dementia or Cognitive Impairment: A Systematic Review and Meta-analysis". JAMA. 323 (19): 1934–1944. doi:10.1001/jama.2020.4249. PMC 7237983. PMID 32427305.
- ^ Arapakis K, Brunner E, French E, McCauley J (October 2021). "Dementia and disadvantage in the USA and England: population-based comparative study". BMJ Open. 11 (10): e045186. doi:10.1136/bmjopen-2020-045186. PMC 8496387. PMID 34615672.
- ^ Daly B, Thompsell A, Sharpling J, Rooney YM, Hillman L, Wanyonyi KL, White S, Gallagher JE (January 2018). "Evidence summary: the relationship between oral health and dementia" (PDF). British Dental Journal. 223 (11): 846–853. doi:10.1038/sj.bdj.2017.992. PMID 29192686. S2CID 19633523.
- ^ Miklossy J (2015). "Historic evidence to support a causal relationship between spirochetal infections and Alzheimer's disease". Frontiers in Aging Neuroscience. 7: 46. doi:10.3389/fnagi.2015.00046. PMC 4399390. PMID 25932012.
- ^ a b c Olsen I, Singhrao SK (September 17, 2015). "Can oral infection be a risk factor for Alzheimer's disease?". Journal of Oral Microbiology. 7: 29143. doi:10.3402/jom.v7.29143. PMC 4575419. PMID 26385886.
- ^ "Can poor oral health lead to dementia?". British Dental Journal. 223 (11): 840. December 2017. doi:10.1038/sj.bdj.2017.1064. PMID 29243693. S2CID 25898592.
- ^ Carter CJ (February 2011). "Alzheimer's disease plaques and tangles: cemeteries of a pyrrhic victory of the immune defence network against herpes simplex infection at the expense of complement and inflammation-mediated neuronal destruction". Neurochemistry International. 58 (3): 301–320. doi:10.1016/j.neuint.2010.12.003. PMID 21167244. S2CID 715832.
- ^ Gibson, GE, Hirsch, JA, Fonzetti, P, et al. (2016) Vitamin B1 (thiamine) and dementia. Ann N Y Acad Sci 1367, 21–30
- ^ Butterworth, RF (2003) Thiamin deficiency and brain disorders. Nutr Res Rev 16, 277–284.
- ^ Hoffman, R. (2016). Thiamine deficiency in the Western diet and dementia risk. British Journal Of Nutrition, 116(1), 188–189.
- ^ a b Dominguez LJ, Barbagallo M (June 2018). "Nutritional prevention of cognitive decline and dementia". Acta Bio Medica: Atenei Parmensis. 89 (2): 276–290. doi:10.23750/abm.v89i2.7401. PMC 6179018. PMID 29957766.
- ^ Goodman B. "Diet Affects Markers of Alzheimer's Disease". WebMD. Retrieved December 13, 2020.
- ^ "Memory loss can be caused by a number of factors, from short term causes such as low blood sugar or medication side effects to long term health issues such as dementia". Diabetes. January 15, 2019. Retrieved December 13, 2020.
- ^ Cao L, Tan L, Wang HF, Jiang T, Zhu XC, Lu H, et al. (November 2016). "Dietary Patterns and Risk of Dementia: a Systematic Review and Meta-Analysis of Cohort Studies". Molecular Neurobiology. 53 (9): 6144–6154. doi:10.1007/s12035-015-9516-4. OCLC 6947867710. PMID 26553347. S2CID 8188716.
- ^ Canevelli M, Lucchini F, Quarata F, Bruno G, Cesari M (March 2016). "Nutrition and Dementia: Evidence for Preventive Approaches?". Nutrients. 8 (3). MDPI: 144. doi:10.3390/nu8030144. OCLC 8147564576. PMC 4808873. PMID 26959055.
- ^ Shannon OM, Ranson JM, Gregory S, Macpherson H, Milte C, Lentjes M, Mulligan A, McEvoy C, Griffiths A, Matu J, Hill TR, Adamson A, Siervo M, Minihane AM, Muniz-Tererra G, Ritchie C, Mathers JC, Llewellyn DJ, Stevenson E (March 2023). "Mediterranean diet adherence is associated with lower dementia risk, independent of genetic predisposition: findings from the UK Biobank prospective cohort study". BMC Med. 21 (1): 81. doi:10.1186/s12916-023-02772-3. PMC 10012551. PMID 36915130. S2CID 257499227.
- ^ Omar SH (June 2019). "Mediterranean and MIND Diets Containing Olive Biophenols Reduces the Prevalence of Alzheimer's Disease". Int J Mol Sci. 20 (11): 2797. doi:10.3390/ijms20112797. PMC 6600544. PMID 31181669.
- ^ Burckhardt M, Herke M, Wustmann T, Watzke S, Langer G, Fink A (April 2016). "Omega-3 fatty acids for the treatment of dementia". The Cochrane Database of Systematic Reviews. 2016 (4): CD009002. doi:10.1002/14651858.CD009002.pub3. PMC 7117565. PMID 27063583.
- ^ Firth J, Teasdale SB, Allott K, Siskind D, Marx W, Cotter J, Veronese N, Schuch F, Smith L, Solmi M, Carvalho AF, Vancampfort D, Berk M, Stubbs B, Sarris J (October 2019). "The efficacy and safety of nutrient supplements in the treatment of mental disorders: a meta-review of meta-analyses of randomized controlled trials". World Psychiatry. 18 (3): 308–324. doi:10.1002/wps.20672. PMC 6732706. PMID 31496103.
- ^ Hafdi M, Hoevenaar-Blom MP, Richard E (November 2021). "Multi-domain interventions for the prevention of dementia and cognitive decline". The Cochrane Database of Systematic Reviews. 2021 (11): CD013572. doi:10.1002/14651858.CD013572.pub2. PMC 8574768. PMID 34748207. S2CID 243846602.
- ^ Schneider LS, Mangialasche F, Andreasen N, Feldman H, Giacobini E, Jones R, et al. (March 2014). "Clinical trials and late-stage drug development for Alzheimer's disease: an appraisal from 1984 to 2014". Journal of Internal Medicine. 275 (3): 251–283. doi:10.1111/joim.12191. PMC 3956752. PMID 24605808.
- ^ Watt JA, Goodarzi Z, Veroniki AA, Nincic V, Khan PA, Ghassemi M, et al. (November 2019). "Comparative Efficacy of Interventions for Aggressive and Agitated Behaviors in Dementia: A Systematic Review and Network Meta-analysis". Annals of Internal Medicine. 171 (9): 633–642. doi:10.7326/M19-0993. PMID 31610547. S2CID 204699972.
- ^ Vandepitte S, Van Den Noortgate N, Putman K, Verhaeghe S, Verdonck C, Annemans L (December 2016). "Effectiveness of respite care in supporting informal caregivers of persons with dementia: a systematic review". International Journal of Geriatric Psychiatry. 31 (12): 1277–1288. doi:10.1002/gps.4504. PMID 27245986. S2CID 3464912.
- ^ a b c d Walsh SC, Murphy E, Devane D, Sampson EL, Connolly S, Carney P, O'Shea E (September 2021). "Palliative care interventions in advanced dementia". The Cochrane Database of Systematic Reviews. 2021 (9): CD011513. doi:10.1002/14651858.CD011513.pub3. PMC 8478014. PMID 34582034.
- ^ Forbes D, Forbes SC, Blake CM, Thiessen EJ, Forbes S (April 2015). "Exercise programs for people with dementia". The Cochrane Database of Systematic Reviews (Submitted manuscript). 132 (4): CD006489. doi:10.1002/14651858.CD006489.pub4. PMC 9426996. PMID 25874613.
- ^ Camp CJ (2010). "Origins of Montessori Programming for Dementia". Non-pharmacological Therapies in Dementia. 1 (2): 163–174. PMC 3600589. PMID 23515663.
- ^ Cheong CY, Tan JA, Foong YL, Koh HM, Chen DZ, Tan JJ, Ng CJ, Yap P (2016). "Creative Music Therapy in an Acute Care Setting for Older Patients with Delirium and Dementia". Dementia and Geriatric Cognitive Disorders Extra. 6 (2): 268–275. doi:10.1159/000445883. PMC 4959431. PMID 27489560.
- ^ Jeon YH, Li Z, Low LF, Chenoweth L, O'Connor D, Beattie E, Liu Z, Brodaty H (August 2015). "The clinical utility of the Cornell Scale for Depression in Dementia as a routine assessment in nursing homes". The American Journal of Geriatric Psychiatry. 23 (8): 784–793. doi:10.1016/j.jagp.2014.08.013. PMID 25256214.
- ^ Jeon YH, Liu Z, Li Z, et al. (November 2016). "Development and Validation of a Short Version of the Cornell Scale for Depression in Dementia for Screening Residents in Nursing Homes". The American Journal of Geriatric Psychiatry. 24 (11): 1007–1016. doi:10.1016/j.jagp.2016.05.012. hdl:1959.4/unsworks_39417. PMID 27538349.
- ^ Harper AE, Rouch S, Leland NE, Turner RL, Mansbach WE, Day CE, Terhorst L (April 2022). "A Systematic Review of Tools Assessing the Perspective of Caregivers of Residents With Dementia". Journal of Applied Gerontology. 41 (4): 1196–1208. doi:10.1177/07334648211028692. PMID 34229505. S2CID 235758241.
- ^ "The WHELD programme for people with dementia helps care home staff deliver person-centred care". NIHR Evidence (Plain English summary). November 26, 2020. doi:10.3310/alert_42713. S2CID 240719455.
- ^ Ballard C, Orrell M, Moniz-Cook E, Woods R, Whitaker R, Corbett A, et al. (July 2020). "Improving mental health and reducing antipsychotic use in people with dementia in care homes: the WHELD research programme including two RCTs". Programme Grants for Applied Research. 8 (6): 1–98. doi:10.3310/pgfar08060. PMID 32721145. S2CID 225489651.
- ^ Woods B, O'Philbin L, Farrell EM, Spector AE, Orrell M (March 2018). "Reminiscence therapy for dementia". The Cochrane Database of Systematic Reviews. 2018 (3): CD001120. doi:10.1002/14651858.CD001120.pub3. PMC 6494367. PMID 29493789.
- ^ Vernooij-Dassen M, Draskovic I, McCleery J, Downs M (November 2011). "Cognitive reframing for carers of people with dementia". The Cochrane Database of Systematic Reviews (11): CD005318. arXiv:0706.4406. doi:10.1002/14651858.CD005318.pub2. hdl:2066/97731. PMID 22071821. S2CID 205178315.
- ^ Neal M, Barton Wright P (2003). "Validation therapy for dementia". The Cochrane Database of Systematic Reviews (3): CD001394. doi:10.1002/14651858.CD001394. PMID 12917907.
- ^ Woods B, Aguirre E, Spector AE, Orrell M (February 2012). "Cognitive stimulation to improve cognitive functioning in people with dementia". The Cochrane Database of Systematic Reviews. 2 (2): CD005562. doi:10.1002/14651858.CD005562.pub2. PMID 22336813. S2CID 7086782.
- ^ a b Möhler R, Renom A, Renom H, Meyer G (August 2020). "Personally tailored activities for improving psychosocial outcomes for people with dementia in community settings". The Cochrane Database of Systematic Reviews. 2020 (8): CD010515. doi:10.1002/14651858.CD010515.pub2. PMC 8094398. PMID 32786083.
- ^ Barker P (2003). Psychiatric and mental health nursing: the craft of caring. London: Arnold. ISBN 978-0-340-81026-2. OCLC 53373798.
- ^ Weitzel T, Robinson S, Barnes MR, et al. (2011). "The special needs of the hospitalized patient with dementia". Medsurg Nursing. 20 (1): 13–18, quiz 19. PMID 21446290.
- ^ Cunningham C (2006). "Understanding challenging behaviour in patients with dementia". Nursing Standard. 20 (47): 42–45. doi:10.7748/ns2006.08.20.47.42.c4477. PMID 16913375.
- ^ a b Dyer SM, Harrison SL, Laver K, Whitehead C, Crotty M (March 2018). "An overview of systematic reviews of pharmacological and non-pharmacological interventions for the treatment of behavioral and psychological symptoms of dementia". International Psychogeriatrics. 30 (3): 295–309. doi:10.1017/S1041610217002344. PMID 29143695.
- ^ van der Steen JT, Smaling HJ, van der Wouden JC, Bruinsma MS, Scholten RJ, Vink AC (July 2018). "Music-based therapeutic interventions for people with dementia". The Cochrane Database of Systematic Reviews. 2018 (7): CD003477. doi:10.1002/14651858.CD003477.pub4. hdl:1874/350441. PMC 6513122. PMID 30033623.
- ^ Osman SE, Tischler V, Schneider J (November 2016). "'Singing for the Brain': A qualitative study exploring the health and well-being benefits of singing for people with dementia and their carers". Dementia. 15 (6): 1326–1339. doi:10.1177/1471301214556291. PMC 5089222. PMID 25425445.
- ^ a b c Johnston B, Narayanasamy M (April 2016). "Exploring psychosocial interventions for people with dementia that enhance personhood and relate to legacy- an integrative review". BMC Geriatrics. 16: 77. doi:10.1186/s12877-016-0250-1. PMC 4820853. PMID 27044417.
- ^ "British hospitals are having a dementia-friendly makeover". The Economist. Retrieved September 19, 2018.
- ^ a b Raj SE, Mackintosh S, Fryer C, Stanley M (January 1, 2021). "Home-Based Occupational Therapy for Adults With Dementia and Their Informal Caregivers: A Systematic Review". The American Journal of Occupational Therapy. 75 (1): 7501205060p1–7501205060p27. doi:10.5014/ajot.2020.040782. PMID 33399054. S2CID 230618534.
- ^ a b Frankenstein LL, Jahn G (April 20, 2020). "Behavioral and Occupational Therapy for Dementia Patients and Caregivers". GeroPsych. 33 (2): 85–100. doi:10.1024/1662-9647/a000225. ISSN 1662-9647. S2CID 219081899.
- ^ Orgeta V, McDonald KR, Poliakoff E, et al. (February 2020). "Cognitive training interventions for dementia and mild cognitive impairment in Parkinson's disease". Cochrane Database of Systematic Reviews. 2020 (2): CD011961. doi:10.1002/14651858.cd011961.pub2. PMC 7043362. PMID 32101639.
- ^ Kudlicka A, Martyr A, Bahar-Fuchs A, et al. (June 29, 2023). Cochrane Dementia and Cognitive Improvement Group (ed.). "Cognitive rehabilitation for people with mild to moderate dementia". Cochrane Database of Systematic Reviews. 2023 (6): CD013388. doi:10.1002/14651858.CD013388.pub2. PMC 10310315. PMID 37389428.
- ^ Möhler R, Calo S, Renom A, Renom H, Meyer G (March 2023). "Personally tailored activities for improving psychosocial outcomes for people with dementia in long-term care". The Cochrane Database of Systematic Reviews. 2023 (3): CD009812. doi:10.1002/14651858.CD009812.pub3. PMC 10010156. PMID 36930048.
- ^ Rafii MS, Aisen PS (February 2009). "Recent developments in Alzheimer's disease therapeutics". BMC Medicine. 7: 7. doi:10.1186/1741-7015-7-7. PMC 2649159. PMID 19228370.
- ^ a b Lleó A, Greenberg SM, Growdon JH (2006). "Current pharmacotherapy for Alzheimer's disease". Annual Review of Medicine. 57 (1): 513–533. doi:10.1146/annurev.med.57.121304.131442. PMID 16409164.
- ^ a b Bond M, Rogers G, Peters J, Anderson R, Hoyle M, Miners A, et al. (2012). "The effectiveness and cost-effectiveness of donepezil, galantamine, rivastigmine and memantine for the treatment of Alzheimer's disease (review of Technology Appraisal No. 111): a systematic review and economic model". Health Technology Assessment. 16 (21): 1–470. doi:10.3310/hta16210. PMC 4780923. PMID 22541366.
- ^ Rodda J, Morgan S, Walker Z (October 2009). "Are cholinesterase inhibitors effective in the management of the behavioral and psychological symptoms of dementia in Alzheimer's disease? A systematic review of randomized, placebo-controlled trials of donepezil, rivastigmine and galantamine". International Psychogeriatrics. 21 (5): 813–824. doi:10.1017/S1041610209990354 (inactive November 1, 2024). PMID 19538824. S2CID 206299435.
{{cite journal}}: CS1 maint: DOI inactive as of November 2024 (link) - ^ Birks J (January 2006). Birks JS (ed.). "Cholinesterase inhibitors for Alzheimer's disease". The Cochrane Database of Systematic Reviews. 2016 (1): CD005593. doi:10.1002/14651858.CD005593. PMC 9006343. PMID 16437532.
- ^ Gill SS, Anderson GM, Fischer HD, Bell CM, Li P, Normand SL, Rochon PA (May 2009). "Syncope and its consequences in patients with dementia receiving cholinesterase inhibitors: a population-based cohort study". Archives of Internal Medicine. 169 (9): 867–873. doi:10.1001/archinternmed.2009.43. PMID 19433698.
- ^ Wang K, Alan J, Page AT, Dimopoulos E, Etherton-Beer C (September 2021). "Anticholinergics and clinical outcomes amongst people with pre-existing dementia: A systematic review". Maturitas. 151. Elsevier BV: 1–14. doi:10.1016/j.maturitas.2021.06.004. PMID 34446273.
- ^ AMDA – The Society for Post-Acute and Long-Term Care Medicine (February 2014), "Ten Things Physicians and Patients Should Question", Choosing Wisely: an initiative of the ABIM Foundation, AMDA – The Society for Post-Acute and Long-Term Care Medicine, archived from the original on April 12, 2015, retrieved April 20, 2015
- ^ Walker Z, Possin KL, Boeve BF, Aarsland D (October 2015). "Lewy body dementias". Lancet (Review). 386 (10004): 1683–1697. doi:10.1016/S0140-6736(15)00462-6. PMC 5792067. PMID 26595642.
- ^ Boot BP (2015). "Comprehensive treatment of dementia with Lewy bodies". Alzheimers Res Ther (Review). 7 (1): 45. doi:10.1186/s13195-015-0128-z. PMC 4448151. PMID 26029267.
- ^ Gomperts SN (April 2016). "Lewy body dementias: Dementia with Lewy bodies and Parkinson disease dementia". Continuum (Minneap Minn) (Review). 22 (2 Dementia): 435–463. doi:10.1212/CON.0000000000000309. PMC 5390937. PMID 27042903.
- ^ a b c d e f g American Geriatrics Society. "Five Things Physicians and Patients Should Question". Choosing Wisely: An Initiative of the ABIM Foundation. Archived from the original on September 1, 2013. Retrieved August 1, 2013.
- ^ American Psychiatric Association (September 2013), "Five Things Physicians and Patients Should Question", Choosing Wisely: an initiative of the ABIM Foundation, American Psychiatric Association, archived from the original on December 3, 2013, retrieved December 30, 2013
- ^ "Dementia: assessment, management and support for people living with dementia and their carers | Guidance and guidelines | NICE". NICE. June 20, 2018. Retrieved December 18, 2018.
- ^ Dyer SM, Laver K, Pond CD, Cumming RG, Whitehead C, Crotty M (December 2016). "Clinical practice guidelines and principles of care for people with dementia in Australia". Australian Family Physician. 45 (12): 884–889. PMID 27903038.
- ^ Declercq T, Petrovic M, Azermai M, Vander Stichele R, De Sutter AI, van Driel ML, Christiaens T (March 2013). "Withdrawal versus continuation of chronic antipsychotic drugs for behavioural and psychological symptoms in older people with dementia" (PDF). The Cochrane Database of Systematic Reviews. 3 (3): CD007726. doi:10.1002/14651858.CD007726.pub2. hdl:1854/LU-3109108. PMID 23543555.
- ^ Raina P, Santaguida P, Ismaila A, Patterson C, Cowan D, Levine M, et al. (March 2008). "Effectiveness of cholinesterase inhibitors and memantine for treating dementia: evidence review for a clinical practice guideline". Annals of Internal Medicine. 148 (5): 379–397. doi:10.7326/0003-4819-148-5-200803040-00009. PMID 18316756.
- ^ Atri A, Shaughnessy LW, Locascio JJ, Growdon JH (2008). "Long-term course and effectiveness of combination therapy in Alzheimer disease". Alzheimer Disease and Associated Disorders. 22 (3): 209–221. doi:10.1097/WAD.0b013e31816653bc. PMC 2718545. PMID 18580597.
- ^ a b c d Kandiah N, Ong PA, Yuda T, Ng LL, Mamun K, Merchant RA, et al. (February 2019). "Treatment of dementia and mild cognitive impairment with or without cerebrovascular disease: Expert consensus on the use of Ginkgo biloba extract, EGb 761®". CNS Neuroscience & Therapeutics. 25 (2): 288–298. doi:10.1111/cns.13095. PMC 6488894. PMID 30648358.
- ^ McKeage K, Lyseng-Williamson KA (2018). "Ginkgo biloba extract EGb 761® in the symptomatic treatment of mild-to-moderate dementia: a profile of its use". Drugs & Therapy Perspectives. 34 (8): 358–366. doi:10.1007/s40267-018-0537-8. PMC 6267544. PMID 30546253.
- ^ Wang M, Peng H, Peng Z, Huang K, Li T, Li L, et al. (September 2020). "Efficacy and safety of ginkgo preparation in patients with vascular dementia: A protocol for systematic review and meta-analysis". Medicine. 99 (37): e22209. doi:10.1097/MD.0000000000022209. PMC 7489658. PMID 32925798.
- ^ Jones HE, Joshi A, Shenkin S, Mead GE (July 2016). "The effect of treatment with selective serotonin reuptake inhibitors in comparison to placebo in the progression of dementia: a systematic review and meta-analysis". Age and Ageing. 45 (4): 448–456. doi:10.1093/ageing/afw053. hdl:20.500.11820/56792c91-31f0-44cb-8ac0-7f50fad8d91e. PMID 27055878.
- ^ Dudas R, Malouf R, McCleery J, Dening T, et al. (Cochrane Dementia and Cognitive Improvement Group) (August 2018). "Antidepressants for treating depression in dementia". The Cochrane Database of Systematic Reviews. 2018 (8): CD003944. doi:10.1002/14651858.CD003944.pub2. PMC 6513376. PMID 30168578.
- ^ Seitz DP, Adunuri N, Gill SS, Gruneir A, Herrmann N, Rochon P (February 2011). "Antidepressants for agitation and psychosis in dementia". The Cochrane Database of Systematic Reviews (2): CD008191. doi:10.1002/14651858.CD008191.pub2. PMID 21328305.
- ^ Malouf R, Grimley Evans J (October 2008). "Folic acid with or without vitamin B12 for the prevention and treatment of healthy elderly and demented people". The Cochrane Database of Systematic Reviews (4): CD004514. doi:10.1002/14651858.CD004514.pub2. PMID 18843658.
- ^ McGuinness B, Craig D, Bullock R, Malouf R, Passmore P (July 2014). "Statins for the treatment of dementia" (PDF). The Cochrane Database of Systematic Reviews. 2014 (7): CD007514. doi:10.1002/14651858.CD007514.pub3. PMC 11112650. PMID 25004278. Archived from the original (PDF) on September 3, 2019. Retrieved September 3, 2019.
- ^ Jongstra S, Harrison JK, Quinn TJ, Richard E (November 2016). "Antihypertensive withdrawal for the prevention of cognitive decline". The Cochrane Database of Systematic Reviews. 2016 (11): CD011971. doi:10.1002/14651858.CD011971.pub2. PMC 6465000. PMID 27802359.
- ^ Page AT, Potter K, Clifford R, McLachlan AJ, Etherton-Beer C (October 2016). "Medication appropriateness tool for co-morbid health conditions in dementia: consensus recommendations from a multidisciplinary expert panel". Internal Medicine Journal. 46 (10): 1189–1197. doi:10.1111/imj.13215. PMC 5129475. PMID 27527376.
- ^ Wang K, Alan J, Page A, Percival M, Etherton-Beer C (2018). "Medication use to manage comorbidities for people with dementia: a systematic review". Journal of Pharmacy Practice and Research. 48 (4): 356–367. doi:10.1002/jppr.1403. ISSN 2055-2335.
- ^ a b Wilfling D, Calo S, Dichter MN, Meyer G, Möhler R, Köpke S (January 2023). "Non-pharmacological interventions for sleep disturbances in people with dementia". The Cochrane Database of Systematic Reviews. 1 (1): CD011881. doi:10.1002/14651858.CD011881.pub2. PMC 9808594. PMID 36594432.
- ^ a b c d McCleery J, Sharpley AL (November 2020). "Pharmacotherapies for sleep disturbances in dementia". The Cochrane Database of Systematic Reviews. 2020 (11): CD009178. doi:10.1002/14651858.CD009178.pub4. PMC 8094738. PMID 33189083.
- ^ American Geriatrics Society 2012 Beers Criteria Update Expert Panel (April 2012). "American Geriatrics Society updated Beers Criteria for potentially inappropriate medication use in older adults". Journal of the American Geriatrics Society. 60 (4): 616–631. doi:10.1111/j.1532-5415.2012.03923.x. PMC 3571677. PMID 22376048.
- ^ Tisher A, Salardini A (April 2019). "A Comprehensive Update on Treatment of Dementia". Seminars in Neurology. 39 (2): 167–178. doi:10.1055/s-0039-1683408. PMID 30925610. S2CID 88474685.
- ^ Lolk A, Gulmann NC (October 2006). "[Psychopharmacological treatment of behavioral and psychological symptoms in dementia]". Ugeskrift for Laeger (in Danish). 168 (40): 3429–3432. PMID 17032610.
- ^ a b c d e Hadjistavropoulos T, Herr K, Turk DC, Fine PG, Dworkin RH, Helme R, Jackson K, Parmelee PA, Rudy TE, Lynn Beattie B, Chibnall JT, Craig KD, Ferrell B, Ferrell B, Fillingim RB, Gagliese L, Gallagher R, Gibson SJ, Harrison EL, Katz B, Keefe FJ, Lieber SJ, Lussier D, Schmader KE, Tait RC, Weiner DK, Williams J (January 2007). "An interdisciplinary expert consensus statement on assessment of pain in older persons". The Clinical Journal of Pain. 23 (1 Suppl): S1–S43. doi:10.1097/AJP.0b013e31802be869. PMID 17179836. S2CID 43777445.
- ^ a b Shega J, Emanuel L, Vargish L, Levine SK, Bursch H, Herr K, Karp JF, Weiner DK (May 2007). "Pain in persons with dementia: complex, common, and challenging". The Journal of Pain. 8 (5): 373–378. doi:10.1016/j.jpain.2007.03.003. PMID 17485039.
- ^ Blyth FM, Cumming R, Mitchell P, Wang JJ (July 2007). "Pain and falls in older people". European Journal of Pain. 11 (5): 564–571. doi:10.1016/j.ejpain.2006.08.001. PMID 17015026. S2CID 27460864.
- ^ Brown C (2009). "Pain, aging and dementia: The crisis is looming, but are we ready?". British Journal of Occupational Therapy. 72 (8): 371–375. doi:10.1177/030802260907200808. S2CID 73245194.
- ^ Herr K, Bjoro K, Decker S (February 2006). "Tools for assessment of pain in nonverbal older adults with dementia: a state-of-the-science review". Journal of Pain and Symptom Management. 31 (2): 170–192. doi:10.1016/j.jpainsymman.2005.07.001. PMID 16488350.
- ^ Stolee P, Hillier LM, Esbaugh J, Bol N, McKellar L, Gauthier N (February 2005). "Instruments for the assessment of pain in older persons with cognitive impairment". Journal of the American Geriatrics Society. 53 (2): 319–326. doi:10.1111/j.1532-5415.2005.53121.x. PMID 15673359. S2CID 21006144.
- ^ AMDA – The Society for Post-Acute and Long-Term Care Medicine (February 2014), "Five Things Physicians and Patients Should Question", Choosing Wisely: an initiative of the ABIM Foundation, AMDA – The Society for Post-Acute and Long-Term Care Medicine, archived from the original on September 13, 2014, retrieved February 10, 2013
- ^ AMDA – The Society for Post-Acute and Long-Term Care Medicine (February 2014), "Five Things Physicians and Patients Should Question", Choosing Wisely: an initiative of the ABIM Foundation, AMDA – The Society for Post-Acute and Long-Term Care Medicine, archived from the original on September 13, 2014, retrieved February 10, 2013, which cites:
- Teno JM, Gozalo PL, Mitchell SL, Kuo S, Rhodes RL, Bynum JP, Mor V (October 2012). "Does feeding tube insertion and its timing improve survival?". Journal of the American Geriatrics Society. 60 (10): 1918–1921. doi:10.1111/j.1532-5415.2012.04148.x. PMC 3470758. PMID 23002947.
- Palecek EJ, Teno JM, Casarett DJ, Hanson LC, Rhodes RL, Mitchell SL (March 2010). "Comfort feeding only: a proposal to bring clarity to decision-making regarding difficulty with eating for persons with advanced dementia". Journal of the American Geriatrics Society. 58 (3): 580–584. doi:10.1111/j.1532-5415.2010.02740.x. PMC 2872797. PMID 20398123.
- Gillick MR, Volandes AE (June 2008). "The standard of caring: why do we still use feeding tubes in patients with advanced dementia?". Journal of the American Medical Directors Association. 9 (5): 364–367. doi:10.1016/j.jamda.2008.03.011. PMID 18519120.
- ^ Mitchell SL, Kiely DK, Lipsitz LA (February 1997). "The risk factors and impact on survival of feeding tube placement in nursing home residents with severe cognitive impairment". Archives of Internal Medicine. 157 (3): 327–332. doi:10.1001/archinte.1997.00440240091014. PMID 9040301.
- ^ Sampson EL, Candy B, Jones L (April 2009). "Enteral tube feeding for older people with advanced dementia". The Cochrane Database of Systematic Reviews. 2009 (2): CD007209. doi:10.1002/14651858.CD007209.pub2. PMC 7182132. PMID 19370678.
- ^ Lockett MA, Templeton ML, Byrne TK, Norcross ED (February 2002). "Percutaneous endoscopic gastrostomy complications in a tertiary-care center". The American Surgeon. 68 (2): 117–120. doi:10.1177/000313480206800202. PMID 11842953. S2CID 43796062.
- ^ Finocchiaro C, Galletti R, Rovera G, Ferrari A, Todros L, Vuolo A, Balzola F (June 1997). "Percutaneous endoscopic gastrostomy: a long-term follow-up". Nutrition. 13 (6): 520–523. doi:10.1016/S0899-9007(97)00030-0. PMID 9263232.
- ^ Mitchell SL, Mor V, Gozalo PL, Servadio JL, Teno JM (August 2016). "Tube Feeding in US Nursing Home Residents With Advanced Dementia, 2000–2014" (PDF). JAMA. 316 (7): 769–770. doi:10.1001/jama.2016.9374. PMC 4991625. PMID 27533163. Archived (PDF) from the original on September 21, 2017.
- ^ Span P (August 29, 2016). "The Decline of Tube Feeding for Dementia Patients". The New York Times. Archived from the original on September 3, 2016. Retrieved August 31, 2016.
- ^ a b Flynn E, Smith CH, Walsh CD, Walshe M (September 2018). "Modifying the consistency of food and fluids for swallowing difficulties in dementia". The Cochrane Database of Systematic Reviews. 2018 (9): CD011077. doi:10.1002/14651858.cd011077.pub2. PMC 6513397. PMID 30251253.
- ^ Forbes D, Forbes SC, Blake CM, Thiessen EJ, Forbes S (April 2015). "Exercise programs for people with dementia". The Cochrane Database of Systematic Reviews. 2015 (4): CD006489. doi:10.1002/14651858.CD006489.pub4. PMC 9426996. PMID 25874613.
- ^ Smith M, Robinson L, Segal J (November 2, 2018). "Preventing Alzheimer's Disease – HelpGuide.org". HelpGuide.org. Retrieved December 13, 2020.
- ^ Van der Roest HG, Wenborn J, Pastink C, Dröes RM, Orrell M (June 2017). "Assistive technology for memory support in dementia". The Cochrane Database of Systematic Reviews. 2017 (6): CD009627. doi:10.1002/14651858.cd009627.pub2. PMC 6481376. PMID 28602027.
- ^ "7 Technological Innovations for Those With Dementia". Alzheimers.net. Retrieved June 28, 2022.
- ^ Anderson M, Menon R, Oak K, Allan L (June 2022). "The use of technology for social interaction by people with dementia: A scoping review". PLOS Digital Health. 1 (6): e0000053. doi:10.1371/journal.pdig.0000053. PMC 9931370. PMID 36812560.
- ^ Viggo Hansen N, Jørgensen T, Ørtenblad L (October 2006). "Massage and touch for dementia". The Cochrane Database of Systematic Reviews. 2006 (4): CD004989. doi:10.1002/14651858.CD004989.pub2. PMC 6823223. PMID 17054228.
- ^ Ball EL, Owen-Booth B, Gray A, Shenkin SD, Hewitt J, McCleery J (August 2020). "Aromatherapy for dementia". The Cochrane Database of Systematic Reviews. 2020 (8): CD003150. doi:10.1002/14651858.CD003150.pub3. PMC 7437395. PMID 32813272.
- ^ Bosnjak Kuharic D, Markovic D, Brkovic T, Jeric Kegalj M, Rubic Z, Vuica Vukasovic A, et al. (September 2021). "Cannabinoids for the treatment of dementia". The Cochrane Database of Systematic Reviews. 2021 (9): CD012820. doi:10.1002/14651858.CD012820.pub2. PMC 8446835. PMID 34532852.
- ^ Sampson EL, Ritchie CW, Lai R, Raven PW, Blanchard MR (March 2005). "A systematic review of the scientific evidence for the efficacy of a palliative care approach in advanced dementia" (PDF). International Psychogeriatrics. 17 (1): 31–40. doi:10.1017/S1041610205001018 (inactive November 1, 2024). PMID 15945590. S2CID 7861568. Archived from the original (PDF) on February 24, 2019.
{{cite journal}}: CS1 maint: DOI inactive as of November 2024 (link) - ^ Van den Block L (October 2014). "The need for integrating palliative care in ageing and dementia policies". European Journal of Public Health. 24 (5): 705–706. doi:10.1093/eurpub/cku084. PMID 24997202.
- ^ van der Steen JT, Radbruch L, Hertogh CM, de Boer ME, Hughes JC, Larkin P, et al. (March 2014). "White paper defining optimal palliative care in older people with dementia: a Delphi study and recommendations from the European Association for Palliative Care". Palliative Medicine. 28 (3): 197–209. doi:10.1177/0269216313493685. hdl:2066/137210. PMID 23828874.
- ^ Birch D, Draper J (May 2008). "A critical literature review exploring the challenges of delivering effective palliative care to older people with dementia" (PDF). Journal of Clinical Nursing. 17 (9): 1144–1163. doi:10.1111/j.1365-2702.2007.02220.x. PMID 18416791.
- ^ Mitchell G, Agnelli J (October 2015). "Person-centred care for people with dementia: Kitwood reconsidered". Nursing Standard. 30 (7): 46–50. doi:10.7748/ns.30.7.46.s47. PMID 26463810.
- ^ a b González-Fraile E, Ballesteros J, Rueda JR, Santos-Zorrozúa B, Solà I, McCleery J (January 2021). "Remotely delivered information, training and support for informal caregivers of people with dementia". The Cochrane Database of Systematic Reviews. 1 (1): CD006440. doi:10.1002/14651858.cd006440.pub3. PMC 8094510. PMID 33417236.
- ^ Dooley B, Ueno H (February 2, 2022). "Where a Thousand Digital Eyes Keep Watch Over the Elderly". The New York Times. ISSN 0362-4331. Retrieved February 6, 2022.
- ^ "The top 10 causes of death". www.who.int. Retrieved August 12, 2024.
- ^ Nichols E, Steinmetz JD, Vollset SE, Fukutaki K, Chalek J, Abd-Allah F, et al. (GBD 2019 Dementia Forecasting Collaborators) (February 2022). "Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: an analysis for the Global Burden of Disease Study 2019". The Lancet. Public Health. 7 (2): e105–e125. doi:10.1016/S2468-2667(21)00249-8. PMC 8810394. PMID 34998485.
- ^ Gale SA, Acar D, Daffner KR (October 2018). "Dementia". Am J Med. 131 (10): 1161–1169. doi:10.1016/j.amjmed.2018.01.022. PMID 29425707. S2CID 240122313.
- ^ a b c d Alzheimer's Disease International (September 2015). "World Alzheimer Report 2015" (PDF). Archived (PDF) from the original on October 9, 2022. Retrieved October 30, 2018.
- ^ Prince M, Jackson J (2009). "World Alzheimer Report 2009". Alzheimer's Disease International: 38. Archived from the original on March 11, 2012. Retrieved March 11, 2012.
- ^ Wang H, Naghavi M, Allen C, Barber RM, Bhutta ZA, Carter A, et al. (GBD 2015 Mortality and Causes of Death Collaborators) (October 2016). "Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: a systematic analysis for the Global Burden of Disease Study 2015". Lancet. 388 (10053): 1459–1544. doi:10.1016/s0140-6736(16)31012-1. PMC 5388903. PMID 27733281.
- ^ Brijnath B, Croy S, Sabates J, Thodis A, Ellis S, de Crespigny F, et al. (2022). "Including ethnic minorities in dementia research: Recommendations from a scoping review". Alzheimer's & Dementia. 8 (1): e12222. doi:10.1002/trc2.12222. PMC 9053375. PMID 35505899.
- ^ Sabayan B, Wyman-Chick KA, Sedaghat S (February 2023). "The Burden of Dementia Spectrum Disorders and Associated Comorbid and Demographic Features". Clinics in Geriatric Medicine. 39 (1): 1–14. doi:10.1016/j.cger.2022.07.001. PMID 36404023. S2CID 253068389.
- ^ Ariana M. Stickel, Andrew C. McKinnon, Stephanie Matijevik, Matthew D. Grilli, John Ruiz, Lee Ryam (February 26, 2021). "Apolipoprotein E ε4 Allele-Based Differences in Brain Volumes Are Largely Uniform Across Late Middle Aged and Older Hispanic/Latino- and Non-Hispanic/Latino Whites Without Dementia". Frontiers in Aging Neuroscience. 13. doi:10.3389/fnagi.2021.627322. PMC 7952627. PMID 33716715.
- ^ a b Sadock BJ, Sadock VA (2008). "Delirium, Dementia, and Amnestic and Other Cobnitive Disorders and Mental Disorders Due to a General Medical Condition". Kaplan & Sadock's concise textbook of clinical psychiatry (3rd ed.). Philadelphia: Wolters Kluwer/Lippincott Williams & Wilkins. p. 52. ISBN 978-0-7817-8746-8.
- ^ Ali M, Talha M, Naseer B, Jaka S, Gunturu S (August 13, 2024). "Divergent Mortality Patterns Associated With Dementia in the United States: 1999–2020". The Primary Care Companion for CNS Disorders. 26 (4): 56364. doi:10.4088/PCC.24m03724. ISSN 2155-7780. PMID 39178013.
- ^ "What causes young-onset dementia? | Alzheimer's Society". alzheimers.org.uk. Retrieved June 28, 2022.
- ^ "What causes young-onset dementia? | Alzheimer's Society". alzheimers.org.uk. Retrieved January 10, 2022.
- ^ Vieira RT, Caixeta L, Machado S, Silva AC, Nardi AE, Arias-Carrión O, Carta MG (June 14, 2013). "Epidemiology of early-onset dementia: a review of the literature". Clinical Practice and Epidemiology in Mental Health. 9: 88–95. doi:10.2174/1745017901309010088. PMC 3715758. PMID 23878613.
- ^ a b Masters CL, Bateman R, Blennow K, Rowe CC, Sperling RA, Cummings JL (October 2015). "Alzheimer's disease". Nature Reviews. Disease Primers. 1: 15056. doi:10.1038/nrdp.2015.56. PMID 27188934. S2CID 20844163.
- ^ Jessop T, Peisah C (July 2021). "Human Rights and Empowerment in Aged Care: Restraint, Consent and Dying with Dignity". International Journal of Environmental Research and Public Health. 18 (15): 7899. doi:10.3390/ijerph18157899. PMC 8345762. PMID 34360196.
- ^ Berrios GE (November 1987). "Dementia during the seventeenth and eighteenth centuries: a conceptual history". Psychological Medicine. 17 (4): 829–837. doi:10.1017/S0033291700000623. PMID 3324141. S2CID 8262492.
- ^ Berchtold NC, Cotman CW (1998). "Evolution in the conceptu-alization of dementia and Alzheimer's disease: Greco-Roman period to the 1960s". Neurobiol Aging. 19 (3): 173–189. doi:10.1016/s0197-4580(98)00052-9. PMID 9661992. S2CID 24808582.
- ^ Bergener M, Reisberg B (1989). Diagnosis and treatment of senile dementia. Berlin: Springer-Verlag. ISBN 0-387-50800-7. OCLC 19455117.
- ^ Xihua J (1989). Diagnosis and Treatment of Senile Dementia: Research Methods and Perspective. Madhav Books (P) Limited, a unit of Serials Publications. p. 38. ISBN 93-80615-34-5.
- ^ a b c Zilka, N., & Novak, M. (2006). The tangled story of Alois Alzheimer. Bratislavske lekarske listy, 107(9–10), 343–345.
- ^ a b c d e f g h Yang HD, Kim DH, Lee SB, Young LD (December 2016). "History of Alzheimer's Disease". Dementia and Neurocognitive Disorders. 15 (4): 115–121. doi:10.12779/dnd.2016.15.4.115. PMC 6428020. PMID 30906352.
- ^ Kolata G (June 17, 2010). "Drug Trials Test Bold Plan to Slow Alzheimer's". The New York Times. Archived from the original on April 9, 2012. Retrieved June 17, 2010.
- ^ Katzman R (April 1976). "Editorial: The prevalence and malignancy of Alzheimer disease. A major killer". Archives of Neurology. 33 (4): 217–218. doi:10.1001/archneur.1976.00500040001001. PMID 1259639.
- ^ "Prevalence by gender in the UK". Dementia Statistics Hub. Retrieved October 4, 2021.
- ^ "Alzheimer's disease – Symptoms and causes". Mayo Clinic. Retrieved October 1, 2021.
- ^ "What is dementia?". Alzheimer's Association. Retrieved August 6, 2018.
Dementia is often incorrectly referred to as "senility" or "senile dementia," which reflects the formerly widespread but incorrect belief that serious mental decline is a normal part of aging.
- ^ Taylor DC. "Dementia". MedicineNet. Retrieved August 6, 2018.
Senile dementia ("senility") is a term that was once used to describe all dementias; this term is no longer used as a diagnosis.
- ^ "Healthfully". Healthfully. Retrieved December 13, 2020.
- ^ "The Relationship Between Schizophrenia and Dementia". Psychology Today. Retrieved December 13, 2020.
- ^ Brodaty H, Donkin M (April 29, 2017). "Family caregivers of people with dementia". Dialogues in Clinical Neuroscience. 11 (2): 217–228. doi:10.31887/DCNS.2009.11.2/hbrodaty. PMC 3181916. PMID 19585957.
- ^ "Most people caring for relatives with dementia experience loneliness". NIHR Evidence (Plain English summary). July 22, 2020. doi:10.3310/alert_40575. S2CID 243269845.
- ^ Victor CR, Rippon I, Quinn C, Nelis SM, Martyr A, Hart N, et al. (July 2021). "The prevalence and predictors of loneliness in caregivers of people with dementia: findings from the IDEAL programme". Aging & Mental Health. 25 (7): 1232–1238. doi:10.1080/13607863.2020.1753014. hdl:10454/17813. PMID 32306759. S2CID 216028843.
- ^ Jia J, Wei C, Chen S, Li F, Tang Y, Qin W, et al. (April 2018). "The cost of Alzheimer's disease in China and re-estimation of costs worldwide". Alzheimer's & Dementia. 14 (4): 483–491. doi:10.1016/j.jalz.2017.12.006. PMID 29433981. S2CID 46762069.
- ^ "National Alzheimer and Dementia Plans Planned Policies and Activities (PDF)" (PDF). London: Alzheimer's Disease International. April 2012. Archived from the original (PDF) on May 18, 2012. Retrieved December 3, 2012.
- ^ "Addressing Alzheimer's and Other Types of Dementia:Israeli National Strategy Summary Document of the Interdisciplinary, Inter-Organizational Group of Experts » Brookdale". Brookdale. Retrieved June 4, 2018.
- ^ Boseley S (March 26, 2012). "Dementia research funding to more than double to £66m by 2015". The Guardian. London. ISSN 0261-3077. OCLC 60623878. Archived from the original on October 20, 2013. Retrieved April 27, 2012.
- ^ O'Dowd A (July 2017). "Dementia is now leading cause of death in women in England". BMJ. 358: j3445. doi:10.1136/bmj.j3445. PMID 28710087. S2CID 29011449. ProQuest 1919058612.
- ^ "British hospitals are having a dementia-friendly makeover". The Economist. Retrieved September 17, 2018.
- ^ "Drivers with dementia a growing problem, MDs warn". CBC News, Canada. September 19, 2007. Archived from the original on October 2, 2007.
- ^ Thompson SB (2009). "Testamentary capacity and cognitive rehabilitation: implications for head-injured and neurologically impaired individuals". Journal of Cognitive Rehabilitation. 27: 11–13. Also published in: Thompson S (September 2010). "Testamentary capacity and cognitive rehabilitation: implications for head-injured and neurologically impaired individuals". Advancing knowledge into the clinical assessment of dementia (Thesis). pp. 372–381.
- ^ "Tackling dementia". Philanthropy magazine. Winter 2016. Archived from the original on February 11, 2016.
- ^ Garvey M (October 22, 2020). "What Happens When TikTok Looks To The Avant-Garde For A Challenge?". NPR. Archived from the original on October 22, 2020. Retrieved April 6, 2021.
- ^ Ezra M (October 23, 2020). "Why Are TikTok Teens Listening to an Album About Dementia?". The New York Times. Archived from the original on October 23, 2020. Retrieved April 21, 2021.
- ^ "Paul Harvey: Composer with dementia inspires £1m donation". BBC News. November 2, 2020. Retrieved November 2, 2020.
- ^ "Alzheimer's Association Celebrity Champions". Alzheimer's Association. Retrieved July 19, 2024.
- ^ "Willis Family Statement | AFTD". February 13, 2023. Retrieved July 19, 2024.
- ^ Rogers K (July 1, 2022). "What Robin Williams' widow wants you to know about the future of Lewy body dementia". CNN. Retrieved July 19, 2024.
- ^ "Doctor: Rosa Parks suffers from dementia". NBC. September 22, 2004.
- ^ "Reagan's Letter Announcing his Alzheimer's Diagnosis". Ronald Reagan. Retrieved July 19, 2024.
- ^ "What to know about Tony Bennett's health struggles, from Alzheimer's to addiction". TODAY.com. July 21, 2023. Retrieved July 19, 2024.
- ^ "Maureen McGovern on Living with Alzheimer's Disease: 'You Go One Day at a Time'". Peoplemag. Retrieved July 19, 2024.
External links
[edit]- "Result List: PM 32016328: EBSCOhost". web.s.ebscohost.com. Retrieved October 3, 2022.
- Alzheimer's Association
- National Institute on Aging – Alzheimer's disease
