Glycoprotein 130
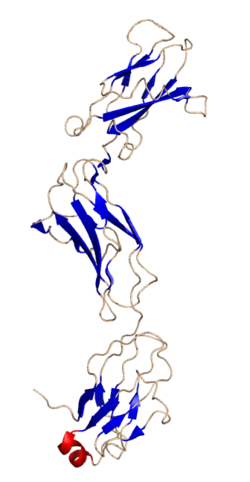
Glycoprotein 130 (also known as gp130, IL6ST, IL6R-beta or CD130) is a transmembrane protein which is the founding member of the class of tall cytokine receptors. It forms one subunit of the type I cytokine receptor within the IL-6 receptor family. It is often referred to as the common gp130 subunit, and is important for signal transduction following cytokine engagement. As with other type I cytokine receptors, gp130 possesses a WSXWS amino acid motif that ensures correct protein folding and ligand binding. It interacts with Janus kinases to elicit an intracellular signal following receptor interaction with its ligand. Structurally, gp130 is composed of five fibronectin type-III domains and one immunoglobulin-like C2-type (immunoglobulin-like) domain in its extracellular portion.[5][6]
Characteristics
[edit]The members of the IL-6 receptor family all complex with gp130 for signal transduction. For example, IL-6 binds to the IL-6 Receptor. The complex of these two proteins then associates with gp130. This complex of 3 proteins then homodimerizes to form a hexameric complex which can produce downstream signals.[7] There are many other proteins which associate with gp130, such as cardiotrophin 1 (CT-1), leukemia inhibitory factor (LIF), ciliary neurotrophic factor (CNTF), oncostatin M (OSM), and IL-11.[8] There are also several other proteins which have structural similarity to gp130 and contain the WSXWS motif and preserved cysteine residues. Members of this group include LIF-R, OSM-R, and G-CSF-R.
Loss of gp130
[edit]gp130 is an important part of many different types of signaling complexes. Inactivation of gp130 is lethal to mice.[9] Homozygous mice who are born show a number of defects including impaired development of the ventricular myocardium. Haematopoietic effects included reduced numbers of stem cells in the spleen and liver.
Signal transduction
[edit]gp130 has no intrinsic tyrosine kinase activity. Instead, it is phosphorylated on tyrosine residues after complexing with other proteins. The phosphorylation leads to association with JAK/Tyk tyrosine kinases and STAT protein transcription factors.[10] In particular, STAT-3 is activated which leads to the activation of many downstream genes. Other pathways activated include RAS and MAPK signaling.
Interactions
[edit]Glycoprotein 130 has been shown to interact with:
- Grb2,[11]
- HER2/neu,[12]
- Janus kinase 1[13][14][15]
- Leukemia inhibitory factor receptor,[16][17]
- PTPN11,[13][18][19]
- SHC1,[20]
- SOCS3,[18] and
- TLE1.[21]
References
[edit]- ^ a b c GRCh38: Ensembl release 89: ENSG00000134352 – Ensembl, May 2017
- ^ a b c GRCm38: Ensembl release 89: ENSMUSG00000021756 – Ensembl, May 2017
- ^ "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ Hibi M, Murakami M, Saito M, Hirano T, Taga T, Kishimoto T (1990). "Molecular cloning and expression of an IL-6 signal transducer, gp130". Cell. 63 (6): 1149–1157. doi:10.1016/0092-8674(90)90411-7. PMID 2261637. S2CID 30852638.
- ^ Bravo J, Staunton D, Heath JK, Jones EY (1998). "Crystal structure of a cytokine-binding region of gp130". EMBO J. 17 (6): 1665–1674. doi:10.1093/emboj/17.6.1665. PMC 1170514. PMID 9501088.
- ^ Murakami M, Hibi M, Nakagawa N, Nakagawa T, Yasukawa K, Yamanishi K, Taga T, Kishimoto T (1993). "IL-6-induced homodimerization of gp130 and associated activation of a tyrosine kinase". Science. 260 (5115): 1808–1810. Bibcode:1993Sci...260.1808M. doi:10.1126/science.8511589. PMID 8511589.
- ^ Kishimoto T, Akira S, Narazaki M, Taga T (1995). "Interleukin-6 family of cytokines and gp130". Blood. 86 (4): 1243–1254. doi:10.1182/blood.V86.4.1243.bloodjournal8641243. PMID 7632928.
- ^ Yoshida K, Taga T, Saito M, Suematsu S, Kumanogoh A, Tanaka T, Fujiwara H, Hirata M, Yamagami T, Nakahata T, Hirabayashi T, Yoneda Y, Tanaka K, Wang WZ, Mori C, Shiota K, Yoshida N, Kishimoto T (1996). "Targeted disruption of gp130, a common signal transducer for the interleukin 6 family of cytokines, leads to myocardial and hematological disorders". Proc. Natl. Acad. Sci. USA. 93 (1): 407–411. Bibcode:1996PNAS...93..407Y. doi:10.1073/pnas.93.1.407. PMC 40247. PMID 8552649.
- ^ Kishimoto T, Taga T, Akira S (1994). "Cytokine signal transduction". Cell. 76 (2): 253–262. doi:10.1016/0092-8674(94)90333-6. PMID 8293462. S2CID 31924790.
- ^ Lee IS, Liu Y, Narazaki M, Hibi M, Kishimoto T, Taga T (January 1997). "Vav is associated with signal transducing molecules gp130, Grb2 and Erk2, and is tyrosine phosphorylated in response to interleukin-6". FEBS Lett. 401 (2–3): 133–7. doi:10.1016/s0014-5793(96)01456-1. PMID 9013873. S2CID 32632406.
- ^ Grant SL, Hammacher A, Douglas AM, Goss GA, Mansfield RK, Heath JK, Begley CG (January 2002). "An unexpected biochemical and functional interaction between gp130 and the EGF receptor family in breast cancer cells". Oncogene. 21 (3): 460–74. doi:10.1038/sj.onc.1205100. PMID 11821958. S2CID 19754641.
- ^ a b Kim H, Baumann H (December 1997). "Transmembrane domain of gp130 contributes to intracellular signal transduction in hepatic cells". J. Biol. Chem. 272 (49): 30741–7. doi:10.1074/jbc.272.49.30741. PMID 9388212.
- ^ Haan C, Is'harc H, Hermanns HM, Schmitz-Van De Leur H, Kerr IM, Heinrich PC, Grötzinger J, Behrmann I (October 2001). "Mapping of a region within the N terminus of Jak1 involved in cytokine receptor interaction". J. Biol. Chem. 276 (40): 37451–8. doi:10.1074/jbc.M106135200. PMID 11468294.
- ^ Haan C, Heinrich PC, Behrmann I (January 2002). "Structural requirements of the interleukin-6 signal transducer gp130 for its interaction with Janus kinase 1: the receptor is crucial for kinase activation". Biochem. J. 361 (Pt 1): 105–11. doi:10.1042/0264-6021:3610105. PMC 1222284. PMID 11742534.
- ^ Timmermann A, Küster A, Kurth I, Heinrich PC, Müller-Newen G (June 2002). "A functional role of the membrane-proximal extracellular domains of the signal transducer gp130 in heterodimerization with the leukemia inhibitory factor receptor". Eur. J. Biochem. 269 (11): 2716–26. doi:10.1046/j.1432-1033.2002.02941.x. PMID 12047380.
- ^ Mosley B, De Imus C, Friend D, Boiani N, Thoma B, Park LS, Cosman D (December 1996). "Dual oncostatin M (OSM) receptors. Cloning and characterization of an alternative signaling subunit conferring OSM-specific receptor activation". J. Biol. Chem. 271 (51): 32635–43. doi:10.1074/jbc.271.51.32635. PMID 8999038.
- ^ a b Lehmann U, Schmitz J, Weissenbach M, Sobota RM, Hortner M, Friederichs K, Behrmann I, Tsiaris W, Sasaki A, Schneider-Mergener J, Yoshimura A, Neel BG, Heinrich PC, Schaper F (January 2003). "SHP2 and SOCS3 contribute to Tyr-759-dependent attenuation of interleukin-6 signaling through gp130". J. Biol. Chem. 278 (1): 661–71. doi:10.1074/jbc.M210552200. PMID 12403768.
- ^ Anhuf D, Weissenbach M, Schmitz J, Sobota R, Hermanns HM, Radtke S, Linnemann S, Behrmann I, Heinrich PC, Schaper F (September 2000). "Signal transduction of IL-6, leukemia-inhibitory factor, and oncostatin M: structural receptor requirements for signal attenuation". J. Immunol. 165 (5): 2535–43. doi:10.4049/jimmunol.165.5.2535. PMID 10946280.
- ^ Giordano V, De Falco G, Chiari R, Quinto I, Pelicci PG, Bartholomew L, Delmastro P, Gadina M, Scala G (May 1997). "Shc mediates IL-6 signaling by interacting with gp130 and Jak2 kinase". J. Immunol. 158 (9): 4097–103. doi:10.4049/jimmunol.158.9.4097. PMID 9126968. S2CID 44339682.
- ^ Liu F, Liu Y, Li D, Zhu Y, Ouyang W, Xie X, Jin B (March 2002). "The transcription co-repressor TLE1 interacted with the intracellular region of gpl30 through its Q domain". Mol. Cell. Biochem. 232 (1–2): 163–7. doi:10.1023/A:1014880813692. PMID 12030375. S2CID 8270300.
Further reading
[edit]- Ip NY, Nye SH, Boulton TG, Davis S, Taga T, Li Y, Birren SJ, Yasukawa K, Kishimoto T, Anderson DJ (1992). "CNTF and LIF act on neuronal cells via shared signaling pathways that involve the IL-6 signal transducing receptor component gp130". Cell. 69 (7): 1121–32. doi:10.1016/0092-8674(92)90634-O. PMID 1617725. S2CID 45816061.
- Hibi M, Murakami M, Saito M, Hirano T, Taga T, Kishimoto T (1991). "Molecular cloning and expression of an IL-6 signal transducer, gp130". Cell. 63 (6): 1149–57. doi:10.1016/0092-8674(90)90411-7. PMID 2261637. S2CID 30852638.
- Taga T, Hibi M, Hirata Y, Yamasaki K, Yasukawa K, Matsuda T, Hirano T, Kishimoto T (1989). "Interleukin-6 triggers the association of its receptor with a possible signal transducer, gp130". Cell. 58 (3): 573–81. doi:10.1016/0092-8674(89)90438-8. PMID 2788034. S2CID 41245022.
- Rodriguez C, Grosgeorge J, Nguyen VC, Gaudray P, Theillet C (1995). "Human gp130 transducer chain gene (IL6ST) is localized to chromosome band 5q11 and possesses a pseudogene on chromosome band 17p11". Cytogenet. Cell Genet. 70 (1–2): 64–7. doi:10.1159/000133993. PMID 7736792.
- Narazaki M, Yasukawa K, Saito T, Ohsugi Y, Fukui H, Koishihara Y, Yancopoulos GD, Taga T, Kishimoto T (1993). "Soluble forms of the interleukin-6 signal-transducing receptor component gp130 in human serum possessing a potential to inhibit signals through membrane-anchored gp130". Blood. 82 (4): 1120–6. doi:10.1182/blood.V82.4.1120.1120. PMID 8353278.
- Davis S, Aldrich TH, Stahl N, Pan L, Taga T, Kishimoto T, Ip NY, Yancopoulos GD (1993). "LIFR beta and gp130 as heterodimerizing signal transducers of the tripartite CNTF receptor". Science. 260 (5115): 1805–8. Bibcode:1993Sci...260.1805D. doi:10.1126/science.8390097. PMID 8390097.
- Murakami M, Hibi M, Nakagawa N, Nakagawa T, Yasukawa K, Yamanishi K, Taga T, Kishimoto T (1993). "IL-6-induced homodimerization of gp130 and associated activation of a tyrosine kinase". Science. 260 (5115): 1808–10. Bibcode:1993Sci...260.1808M. doi:10.1126/science.8511589. PMID 8511589.
- Sharkey AM, Dellow K, Blayney M, Macnamee M, Charnock-Jones S, Smith SK (1996). "Stage-specific expression of cytokine and receptor messenger ribonucleic acids in human preimplantation embryos". Biol. Reprod. 53 (4): 974–81. doi:10.1095/biolreprod53.4.974. PMID 8547494.
- Mosley B, De Imus C, Friend D, Boiani N, Thoma B, Park LS, Cosman D (1997). "Dual oncostatin M (OSM) receptors. Cloning and characterization of an alternative signaling subunit conferring OSM-specific receptor activation". J. Biol. Chem. 271 (51): 32635–43. doi:10.1074/jbc.271.51.32635. PMID 8999038.
- Lee IS, Liu Y, Narazaki M, Hibi M, Kishimoto T, Taga T (1997). "Vav is associated with signal transducing molecules gp130, Grb2 and Erk2, and is tyrosine phosphorylated in response to interleukin-6". FEBS Lett. 401 (2–3): 133–7. doi:10.1016/S0014-5793(96)01456-1. PMID 9013873. S2CID 32632406.
- Auguste P, Guillet C, Fourcin M, Olivier C, Veziers J, Pouplard-Barthelaix A, Gascan H (1997). "Signaling of type II oncostatin M receptor". J. Biol. Chem. 272 (25): 15760–4. doi:10.1074/jbc.272.25.15760. PMID 9188471.
- Schiemann WP, Bartoe JL, Nathanson NM (1997). "Box 3-independent signaling mechanisms are involved in leukemia inhibitory factor receptor alpha- and gp130-mediated stimulation of mitogen-activated protein kinase. Evidence for participation of multiple signaling pathways which converge at Ras". J. Biol. Chem. 272 (26): 16631–6. doi:10.1074/jbc.272.26.16631. PMID 9195977.
- Diamant M, Rieneck K, Mechti N, Zhang XG, Svenson M, Bendtzen K, Klein B (1997). "Cloning and expression of an alternatively spliced mRNA encoding a soluble form of the human interleukin-6 signal transducer gp130". FEBS Lett. 412 (2): 379–84. doi:10.1016/S0014-5793(97)00750-3. PMID 9256256. S2CID 12420499.
- Koshelnick Y, Ehart M, Hufnagl P, Heinrich PC, Binder BR (1997). "Urokinase receptor is associated with the components of the JAK1/STAT1 signaling pathway and leads to activation of this pathway upon receptor clustering in the human kidney epithelial tumor cell line TCL-598". J. Biol. Chem. 272 (45): 28563–7. doi:10.1074/jbc.272.45.28563. PMID 9353320.
- Kim H, Baumann H (1998). "Transmembrane domain of gp130 contributes to intracellular signal transduction in hepatic cells". J. Biol. Chem. 272 (49): 30741–7. doi:10.1074/jbc.272.49.30741. PMID 9388212.
- Bravo J, Staunton D, Heath JK, Jones EY (1998). "Crystal structure of a cytokine-binding region of gp130". EMBO J. 17 (6): 1665–74. doi:10.1093/emboj/17.6.1665. PMC 1170514. PMID 9501088.
- Barton VA, Hudson KR, Heath JK (1999). "Identification of three distinct receptor binding sites of murine interleukin-11". J. Biol. Chem. 274 (9): 5755–61. doi:10.1074/jbc.274.9.5755. PMID 10026196.
- Hirota H, Chen J, Betz UA, Rajewsky K, Gu Y, Ross J, Müller W, Chien KR (1999). "Loss of a gp130 cardiac muscle cell survival pathway is a critical event in the onset of heart failure during biomechanical stress". Cell. 97 (2): 189–98. doi:10.1016/S0092-8674(00)80729-1. PMID 10219240. S2CID 17955799.
- Tacken I, Dahmen H, Boisteau O, Minvielle S, Jacques Y, Grötzinger J, Küster A, Horsten U, Blanc C, Montero-Julian FA, Heinrich PC, Müller-Newen G (1999). "Definition of receptor binding sites on human interleukin-11 by molecular modeling-guided mutagenesis". Eur. J. Biochem. 265 (2): 645–55. doi:10.1046/j.1432-1327.1999.00755.x. PMID 10504396.
- Chung TD, Yu JJ, Kong TA, Spiotto MT, Lin JM (2000). "Interleukin-6 activates phosphatidylinositol-3 kinase, which inhibits apoptosis in human prostate cancer cell lines". Prostate. 42 (1): 1–7. doi:10.1002/(SICI)1097-0045(20000101)42:1<1::AID-PROS1>3.0.CO;2-Y. PMID 10579793. S2CID 86404104.
External links
[edit]- Cytokine+Receptor+gp130 at the U.S. National Library of Medicine Medical Subject Headings (MeSH)