High-performance liquid chromatography

High-performance liquid chromatography (HPLC), formerly referred to as high-pressure liquid chromatography, is a technique in analytical chemistry used to separate, identify, and quantify specific components in mixtures. The mixtures can originate from food, chemicals, pharmaceuticals,[1] biological, environmental and agriculture, etc., which have been dissolved into liquid solutions.[citation needed]
It relies on high pressure pumps, which deliver mixtures of various solvents, called the mobile phase, which flows through the system, collecting the sample mixture on the way, delivering it into a cylinder, called the column, filled with solid particles, made of adsorbent material, called the stationary phase.[2]
Each component in the sample interacts differently with the adsorbent material, causing different migration rates for each component.[3] These different rates lead to separation as the species flow out of the column into a specific detector such as UV detectors. The output of the detector is a graph, called a chromatogram. Chromatograms are graphical representations of the signal intensity versus time or volume, showing peaks, which represent components of the sample. Each sample appears in its respective time, called its retention time, having area proportional to its amount.[2]
HPLC is widely used for manufacturing (e.g., during the production process of pharmaceutical and biological products),[4][5] legal (e.g., detecting performance enhancement drugs in urine),[6] research (e.g., separating the components of a complex biological sample, or of similar synthetic chemicals from each other), and medical (e.g., detecting vitamin D levels in blood serum) purposes.[7]
Chromatography can be described as a mass transfer process involving adsorption and/or partition. As mentioned, HPLC relies on pumps to pass a pressurized liquid and a sample mixture through a column filled with adsorbent, leading to the separation of the sample components. The active component of the column, the adsorbent, is typically a granular material made of solid particles (e.g., silica, polymers, etc.), 1.5–50 μm in size, on which various reagents can be bonded.[8][9] The components of the sample mixture are separated from each other due to their different degrees of interaction with the adsorbent particles. The pressurized liquid is typically a mixture of solvents (e.g., water, buffers, acetonitrile and/or methanol) and is referred to as a "mobile phase". Its composition and temperature play a major role in the separation process by influencing the interactions taking place between sample components and adsorbent.[10] These interactions are physical in nature, such as hydrophobic (dispersive), dipole–dipole and ionic, most often a combination.[11][12]
Operation
[edit]The liquid chromatograph is complex[13] and has sophisticated and delicate technology. In order to properly operate the system, there should be a minimum basis for understanding of how the device performs the data processing to avoid incorrect data and distorted results.[14][15][16]
HPLC is distinguished from traditional ("low pressure") liquid chromatography because operational pressures are significantly higher (around 50–1400 bar), while ordinary liquid chromatography typically relies on the force of gravity to pass the mobile phase through the packed column. Due to the small sample amount separated in analytical HPLC, typical column dimensions are 2.1–4.6 mm diameter, and 30–250 mm length. Also HPLC columns are made with smaller adsorbent particles (1.5–50 μm in average particle size). This gives HPLC superior resolving power (the ability to distinguish between compounds) when separating mixtures, which makes it a popular chromatographic technique.[citation needed]
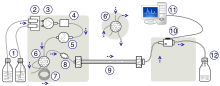
The schematic of an HPLC instrument typically includes solvents' reservoirs, one or more pumps, a solvent-degasser, a sampler, a column, and a detector. The solvents are prepared in advance according to the needs of the separation, they pass through the degasser to remove dissolved gasses, mixed to become the mobile phase, then flow through the sampler, which brings the sample mixture into the mobile phase stream, which then carries it into the column. The pumps deliver the desired flow and composition of the mobile phase through the stationary phase inside the column, then directly into a flow-cell inside the detector. The detector generates a signal proportional to the amount of sample component emerging from the column, hence allowing for quantitative analysis of the sample components. The detector also marks the time of emergence, the retention time, which serves for initial identification of the component. More advanced detectors, provide also additional information, specific to the analyte's characteristics, such as UV-VIS spectrum or mass spectrum, which can provide insight on its structural features. These detectors are in common use, such as UV/Vis, photodiode array (PDA) / diode array detector and mass spectrometry detector.[citation needed]
A digital microprocessor and user software control the HPLC instrument and provide data analysis. Some models of mechanical pumps in an HPLC instrument can mix multiple solvents together at a ratios changing in time, generating a composition gradient in the mobile phase. Most HPLC instruments also have a column oven that allows for adjusting the temperature at which the separation is performed.[citation needed]
The sample mixture to be separated and analyzed is introduced, in a discrete small volume (typically microliters), into the stream of mobile phase percolating through the column. The components of the sample move through the column, each at a different velocity, which are a function of specific physical interactions with the adsorbent, the stationary phase. The velocity of each component depends on its chemical nature, on the nature of the stationary phase (inside the column) and on the composition of the mobile phase. The time at which a specific analyte elutes (emerges from the column) is called its retention time. The retention time, measured under particular conditions, is an identifying characteristic of a given analyte.[citation needed]
Many different types of columns are available, filled with adsorbents varying in particle size, porosity, and surface chemistry. The use of smaller particle size packing materials requires the use of higher operational pressure ("backpressure") and typically improves chromatographic resolution (the degree of peak separation between consecutive analytes emerging from the column). Sorbent particles may be ionic, hydrophobic or polar in nature.[citation needed]
The most common mode of liquid chromatography is reversed phase, whereby the mobile phases used, include any miscible combination of water or buffers with various organic solvents (the most common are acetonitrile and methanol). Some HPLC techniques use water-free mobile phases (see normal-phase chromatography below). The aqueous component of the mobile phase may contain acids (such as formic, phosphoric or trifluoroacetic acid) or salts to assist in the separation of the sample components. The composition of the mobile phase may be kept constant ("isocratic elution mode") or varied ("gradient elution mode") during the chromatographic analysis. Isocratic elution is typically effective in the separation of simple mixtures. Gradient elution is required for complex mixtures, with varying interactions with the stationary and mobile phases. This is the reason why in gradient elution the composition of the mobile phase is varied typically from low to high eluting strength. The eluting strength of the mobile phase is reflected by analyte retention times, as the high eluting strength speeds up the elution (resulting in shortening of retention times). For example, a typical gradient profile in reversed phase chromatography for might start at 5% acetonitrile (in water or aqueous buffer) and progress linearly to 95% acetonitrile over 5–25 minutes. Periods of constant mobile phase composition (plateau) may be also part of a gradient profile. For example, the mobile phase composition may be kept constant at 5% acetonitrile for 1–3 min, followed by a linear change up to 95% acetonitrile.[citation needed]
The chosen composition of the mobile phase depends on the intensity of interactions between various sample components ("analytes") and stationary phase (e.g., hydrophobic interactions in reversed-phase HPLC). Depending on their affinity for the stationary and mobile phases, analytes partition between the two during the separation process taking place in the column. This partitioning process is similar to that which occurs during a liquid–liquid extraction but is continuous, not step-wise.[citation needed]
In the example using a water/acetonitrile gradient, the more hydrophobic components will elute (come off the column) later, then, once the mobile phase gets richer in acetonitrile (i.e., in a mobile phase becomes higher eluting solution), their elution speeds up.[citation needed]
The choice of mobile phase components, additives (such as salts or acids) and gradient conditions depends on the nature of the column and sample components. Often a series of trial runs is performed with the sample in order to find the HPLC method which gives adequate separation.[citation needed]
History and development
[edit]Prior to HPLC, scientists used benchtop column liquid chromatographic techniques. Liquid chromatographic systems were largely inefficient due to the flow rate of solvents being dependent on gravity. Separations took many hours, and sometimes days to complete. Gas chromatography (GC) at the time was more powerful than liquid chromatography (LC), however, it was obvious that gas phase separation and analysis of very polar high molecular weight biopolymers was impossible.[17] GC was ineffective for many life science and health applications for biomolecules, because they are mostly non-volatile and thermally unstable at the high temperatures of GC.[18] As a result, alternative methods were hypothesized which would soon result in the development of HPLC.[citation needed]
Following on the seminal work of Martin and Synge in 1941, it was predicted by Calvin Giddings,[19] Josef Huber, and others in the 1960s that LC could be operated in the high-efficiency mode by reducing the packing-particle diameter substantially below the typical LC (and GC) level of 150 μm and using pressure to increase the mobile phase velocity.[17] These predictions underwent extensive experimentation and refinement throughout the 60s into the 70s until these very days.[20] Early developmental research began to improve LC particles, for example the historic Zipax, a superficially porous particle.[21]
The 1970s brought about many developments in hardware and instrumentation. Researchers began using pumps and injectors to make a rudimentary design of an HPLC system.[22] Gas amplifier pumps were ideal because they operated at constant pressure and did not require leak-free seals or check valves for steady flow and good quantitation.[18] Hardware milestones were made at Dupont IPD (Industrial Polymers Division) such as a low-dwell-volume gradient device being utilized as well as replacing the septum injector with a loop injection valve.[18]
While instrumentation developments were important, the history of HPLC is primarily about the history and evolution of particle technology.[18][23] After the introduction of porous layer particles, there has been a steady trend to reduced particle size to improve efficiency.[18] However, by decreasing particle size, new problems arose. The practical disadvantages stem from the excessive pressure drop needed to force mobile fluid through the column and the difficulty of preparing a uniform packing of extremely fine materials.[24] Every time particle size is reduced significantly, another round of instrument development usually must occur to handle the pressure.[20][18]
Types
[edit]Partition chromatography
[edit]
Partition chromatography was one of the first kinds of chromatography that chemists developed, and is barely used these days.[25] The partition coefficient principle has been applied in paper chromatography, thin layer chromatography, gas phase and liquid–liquid separation applications. The 1952 Nobel Prize in chemistry was earned by Archer John Porter Martin and Richard Laurence Millington Synge for their development of the technique, which was used for their separation of amino acids.[26] Partition chromatography uses a retained solvent, on the surface or within the grains or fibers of an "inert" solid supporting matrix as with paper chromatography; or takes advantage of some coulombic and/or hydrogen donor interaction with the stationary phase. Analyte molecules partition between a liquid stationary phase and the eluent. Just as in hydrophilic interaction chromatography (HILIC; a sub-technique within HPLC), this method separates analytes based on differences in their polarity. HILIC most often uses a bonded polar stationary phase and a mobile phase made primarily of acetonitrile with water as the strong component. Partition HPLC has been used historically on unbonded silica or alumina supports. Each works effectively for separating analytes by relative polar differences. HILIC bonded phases have the advantage of separating acidic, basic and neutral solutes in a single chromatographic run.[27]
The polar analytes diffuse into a stationary water layer associated with the polar stationary phase and are thus retained. The stronger the interactions between the polar analyte and the polar stationary phase (relative to the mobile phase) the longer the elution time. The interaction strength depends on the functional groups part of the analyte molecular structure, with more polarized groups (e.g., hydroxyl-) and groups capable of hydrogen bonding inducing more retention. Coulombic (electrostatic) interactions can also increase retention. Use of more polar solvents in the mobile phase will decrease the retention time of the analytes, whereas more hydrophobic solvents tend to increase retention times.[citation needed]
Normal–phase chromatography
[edit]Normal–phase chromatography was one of the first kinds of HPLC that chemists developed, but has decreased in use over the last decades. Also known as normal-phase HPLC (NP-HPLC), this method separates analytes based on their affinity for a polar stationary surface such as silica; hence it is based on analyte ability to engage in polar interactions (such as hydrogen-bonding or dipole-dipole type of interactions) with the sorbent surface. NP-HPLC uses a non-polar, non-aqueous mobile phase (e.g., chloroform), and works effectively for separating analytes readily soluble in non-polar solvents. The analyte associates with and is retained by the polar stationary phase. Adsorption strengths increase with increased analyte polarity. The interaction strength depends not only on the functional groups present in the structure of the analyte molecule, but also on steric factors. The effect of steric hindrance on interaction strength allows this method to resolve (separate) structural isomers.[citation needed]
The use of more polar solvents in the mobile phase will decrease the retention time of analytes, whereas more hydrophobic solvents tend to induce slower elution (increased retention times). Very polar solvents such as traces of water in the mobile phase tend to adsorb to the solid surface of the stationary phase forming a stationary bound (water) layer which is considered to play an active role in retention. This behavior is somewhat peculiar to normal phase chromatography because it is governed almost exclusively by an adsorptive mechanism (i.e., analytes interact with a solid surface rather than with the solvated layer of a ligand attached to the sorbent surface; see also reversed-phase HPLC below). Adsorption chromatography is still somewhat used for structural isomer separations in both column and thin-layer chromatography formats on activated (dried) silica or alumina supports.[citation needed]
Partition- and NP-HPLC fell out of favor in the 1970s with the development of reversed-phase HPLC because of poor reproducibility of retention times due to the presence of a water or protic organic solvent layer on the surface of the silica or alumina chromatographic media. This layer changes with any changes in the composition of the mobile phase (e.g., moisture level) causing drifting retention times.[citation needed]
Recently, partition chromatography has become popular again with the development of Hilic bonded phases which demonstrate improved reproducibility, and due to a better understanding of the range of usefulness of the technique.
Displacement chromatography
[edit]The use of displacement chromatography is rather limited, and is mostly used for preparative chromatography. The basic principle is based on a molecule with a high affinity for the chromatography matrix (the displacer) which is used to compete effectively for binding sites, and thus displace all molecules with lesser affinities.[28] There are distinct differences between displacement and elution chromatography. In elution mode, substances typically emerge from a column in narrow, Gaussian peaks. Wide separation of peaks, preferably to baseline, is desired in order to achieve maximum purification. The speed at which any component of a mixture travels down the column in elution mode depends on many factors. But for two substances to travel at different speeds, and thereby be resolved, there must be substantial differences in some interaction between the biomolecules and the chromatography matrix. Operating parameters are adjusted to maximize the effect of this difference. In many cases, baseline separation of the peaks can be achieved only with gradient elution and low column loadings. Thus, two drawbacks to elution mode chromatography, especially at the preparative scale, are operational complexity, due to gradient solvent pumping, and low throughput, due to low column loadings. Displacement chromatography has advantages over elution chromatography in that components are resolved into consecutive zones of pure substances rather than "peaks". Because the process takes advantage of the nonlinearity of the isotherms, a larger column feed can be separated on a given column with the purified components recovered at significantly higher concentration.[citation needed]
Reversed-phase liquid chromatography (RP-LC)
[edit]
Reversed phase HPLC (RP-HPLC)[29] is the most widespread mode of chromatography. It has a non-polar stationary phase and an aqueous, moderately polar mobile phase. In the reversed phase methods, the substances are retained in the system the more hydrophobic they are. For the retention of organic materials, the stationary phases, packed inside the columns, are consisted mainly of porous granules of silica gel in various shapes, mainly spherical, at different diameters (1.5, 2, 3, 5, 7, 10 um), with varying pore diameters (60, 100, 150, 300, A), on whose surface are chemically bound various hydrocarbon ligands such as C3, C4, C8, C18. There are also polymeric hydrophobic particles that serve as stationary phases, when solutions at extreme pH are needed, or hybrid silica, polymerized with organic substances. The longer the hydrocarbon ligand on the stationary phase, the longer the sample components can be retained. Most of the current methods of separation of biomedical materials use C-18 type of columns, sometimes called by a trade names such as ODS (octadecylsilane) or RP-18 (Reversed Phase 18).
The most common RP stationary phases are based on a silica support, which is surface-modified by bonding RMe2SiCl, where R is a straight chain alkyl group such as C18H37 or C8H17.
With such stationary phases, retention time is longer for lipophylic molecules, whereas polar molecules elute more readily (emerge early in the analysis). A chromatographer can increase retention times by adding more water to the mobile phase, thereby making the interactions of the hydrophobic analyte with the hydrophobic stationary phase relatively stronger. Similarly, an investigator can decrease retention time by adding more organic solvent to the mobile phase. RP-HPLC is so commonly used among the biologists and life science users, therefore it is often incorrectly referred to as just "HPLC" without further specification. The pharmaceutical industry also regularly employs RP-HPLC to qualify drugs before their release.[citation needed]
RP-HPLC operates on the principle of hydrophobic interactions, which originates from the high symmetry in the dipolar water structure and plays the most important role in all processes in life science. RP-HPLC allows the measurement of these interactive forces. The binding of the analyte to the stationary phase is proportional to the contact surface area around the non-polar segment of the analyte molecule upon association with the ligand on the stationary phase. This solvophobic effect is dominated by the force of water for "cavity-reduction" around the analyte and the C18-chain versus the complex of both. The energy released in this process is proportional to the surface tension of the eluent (water: 7.3×10−6 J/cm2, methanol: 2.2×10−6 J/cm2) and to the hydrophobic surface of the analyte and the ligand respectively. The retention can be decreased by adding a less polar solvent (methanol, acetonitrile) into the mobile phase to reduce the surface tension of water. Gradient elution uses this effect by automatically reducing the polarity and the surface tension of the aqueous mobile phase during the course of the analysis.
Structural properties of the analyte molecule can play an important role in its retention characteristics. In theory, an analyte with a larger hydrophobic surface area (C–H, C–C, and generally non-polar atomic bonds, such as S-S and others) can be retained longer as it does not interact with the water structure. On the other hand, analytes with higher polar surface area (as a result of the presence of polar groups, such as -OH, -NH2, COO− or -NH3+ in their structure) are less retained, as they are better integrated into water. The interactions with the stationary phase can also affected by steric effects, or exclusion effects, whereby a component of very large molecule may have only restricted access to the pores of the stationary phase, where the interactions with surface ligands (alkyl chains) take place. Such surface hindrance typically results in less retention.
Retention time increases with more hydrophobic (non-polar) surface area of the molecules. For example, branched chain compounds can elute more rapidly than their corresponding linear isomers because their overall surface area is lower. Similarly organic compounds with single C–C bonds frequently elute later than those with a C=C or even triple bond, as the double or triple bond makes the molecule more compact than a single C–C bond.
Another important factor is the mobile phase pH since it can change the hydrophobic character of the ionizable analyte. For this reason most methods use a buffering agent, such as sodium phosphate, to control the pH. Buffers serve multiple purposes: control of pH which affects the ionization state of the ionizable analytes, affect the charge upon the ionizable silica surface of the stationary phase in between the bonded phase linands, and in some cases even act as ion pairing agents to neutralize analyte charge. Ammonium formate is commonly added in mass spectrometry to improve detection of certain analytes by the formation of analyte-ammonium adducts. A volatile organic acid such as acetic acid, or most commonly formic acid, is often added to the mobile phase if mass spectrometry is used to analyze the column effluents.
Trifluoroacetic acid (TFA) as additive to the mobile phase is widely used for complex mixtures of biomedical samples, mostly peptides and proteins, using mostly UV based detectors. They are rarely used in mass spectrometry methods, due to residues it can leave in the detector and solvent delivery system, which interfere with the analysis and detection. However, TFA can be highly effective in improving retention of analytes such as carboxylic acids, in applications utilizing other detectors such as UV-VIS, as it is a fairly strong organic acid. The effects of acids and buffers vary by application but generally improve chromatographic resolution when dealing with ionizable components.
Reversed phase columns are quite difficult to damage compared to normal silica columns, thanks to the shielding effect of the bonded hydrophobic ligands; however, most reversed phase columns consist of alkyl derivatized silica particles, and are prone to hydrolysis of the silica at extreme pH conditions in the mobile phase. Most types of RP columns should not be used with aqueous bases as these will hydrolyze the underlying silica particle and dissolve it. There are selected brands of hybrid or enforced silica based particles of RP columns which can be used at extreme pH conditions. The use of extreme acidic conditions is also not recommended, as they also might hydrolyzed as well as corrode the inside walls of the metallic parts of the HPLC equipment.
As a rule, in most cases RP-HPLC columns should be flushed with clean solvent after use to remove residual acids or buffers, and stored in an appropriate composition of solvent. Some biomedical applications require non metallic environment for the optimal separation. For such sensitive cases there is a test for the metal content of a column is to inject a sample which is a mixture of 2,2'- and 4,4'-bipyridine. Because the 2,2'-bipy can chelate the metal, the shape of the peak for the 2,2'-bipy will be distorted (tailed) when metal ions are present on the surface of the silica.[citation needed]..
Size-exclusion chromatography
[edit]Size-exclusion chromatography (SEC)[30] separates polymer molecules and biomolecules based on differences in their molecular size (actually by a particle's Stokes radius). The separation process is based on the ability of sample molecules to permeate through the pores of gel spheres, packed inside the column, and is dependent on the relative size of analyte molecules and the respective pore size of the absorbent. The process also relies on the absence of any interactions with the packing material surface.
Two types of SEC are usually termed:
- Gel permeation chromatography (GPC)—separation of synthetic polymers (aqueous or organic soluble). GPC is a powerful technique for polymer characterization using primarily organic solvents.
- Gel filtration chromatography (GFC)—separation of water-soluble biopolymers. GFC uses primarily aqueous solvents (typically for aqueous soluble biopolymers, such as proteins, etc.).
The separation principle in SEC is based on the fully, or partially penetrating of the high molecular weight substances of the sample into the porous stationary-phase particles during their transport through column. The mobile-phase eluent is selected in such a way that it totally prevents interactions with the stationary phase's surface. Under these conditions, the smaller the size of the molecule, the more it is able to penetrate inside the pore space and the movement through the column takes longer. On the other hand, the bigger the molecular size, the higher the probability the molecule will not fully penetrate the pores of the stationary phase, and even travel around them, thus, will be eluted earlier. The molecules are separated in order of decreasing molecular weight, with the largest molecules eluting from the column first and smaller molecules eluting later. Molecules larger than the pore size do not enter the pores at all, and elute together as the first peak in the chromatogram and this is called total exclusion volume which defines the exclusion limit for a particular column. Small molecules will permeate fully through the pores of the stationary phase particles and will be eluted last, marking the end of the chromatogram, and may appear as a total penetration marker.
In biomedical sciences it is generally considered as a low resolution chromatography and thus it is often reserved for the final, "polishing" step of the purification. It is also useful for determining the tertiary structure and quaternary structure of purified proteins. SEC is used primarily for the analysis of large molecules such as proteins or polymers. SEC works also in a preparative way by trapping the smaller molecules in the pores of a particles. The larger molecules simply pass by the pores as they are too large to enter the pores. Larger molecules therefore flow through the column quicker than smaller molecules: that is, the smaller the molecule, the longer the retention time.
This technique is widely used for the molecular weight determination of polysaccharides. SEC is the official technique (suggested by European pharmacopeia) for the molecular weight comparison of different commercially available low-molecular weight heparins.[31]
Ion-exchange chromatography
[edit]Ion-exchange chromatography (IEC) or ion chromatography (IC)[32] is an analytical technique for the separation and determination of ionic solutes in aqueous samples from environmental and industrial origins such as metal industry, industrial waste water, in biological systems, pharmaceutical samples, food, etc. Retention is based on the attraction between solute ions and charged sites bound to the stationary phase. Solute ions charged the same as the ions on the column are repulsed and elute without retention, while solute ions charged oppositely to the charged sites of the column are retained on it. Solute ions that are retained on the column can be eluted from it by changing the mobile phase composition, such as increasing its salt concentration and pH or increasing the column temperature, etc.
Types of ion exchangers include polystyrene resins, cellulose and dextran ion exchangers (gels), and controlled-pore glass or porous silica gel. Polystyrene resins allow cross linkage, which increases the stability of the chain. Higher cross linkage reduces swerving, which increases the equilibration time and ultimately improves selectivity. Cellulose and dextran ion exchangers possess larger pore sizes and low charge densities making them suitable for protein separation.
In general, ion exchangers favor the binding of ions of higher charge and smaller radius.
An increase in counter ion (with respect to the functional groups in resins) concentration reduces the retention time, as it creates a strong competition with the solute ions. A decrease in pH reduces the retention time in cation exchange while an increase in pH reduces the retention time in anion exchange. By lowering the pH of the solvent in a cation exchange column, for instance, more hydrogen ions are available to compete for positions on the anionic stationary phase, thereby eluting weakly bound cations.
This form of chromatography is widely used in the following applications: water purification, preconcentration of trace components, ligand-exchange chromatography, ion-exchange chromatography of proteins, high-pH anion-exchange chromatography of carbohydrates and oligosaccharides, and others.
Bioaffinity chromatography
[edit]High performance affinity chromatography (HPAC)[33] works by passing a sample solution through a column packed with a stationary phase that contains an immobilized biologically active ligand. The ligand is in fact a substrate that has a specific binding affinity for the target molecule in the sample solution. The target molecule binds to the ligand, while the other molecules in the sample solution pass through the column, having little or no retention. The target molecule is then eluted from the column using a suitable elution buffer.
This chromatographic process relies on the capability of the bonded active substances to form stable, specific, and reversible complexes thanks to their biological recognition of certain specific sample components. The formation of these complexes involves the participation of common molecular forces such as the Van der Waals interaction, electrostatic interaction, dipole-dipole interaction, hydrophobic interaction, and the hydrogen bond. An efficient, biospecific bond is formed by a simultaneous and concerted action of several of these forces in the complementary binding sites.
Aqueous normal-phase chromatography
[edit]Aqueous normal-phase chromatography (ANP) is also called hydrophilic interaction liquid chromatography (HILIC).[34] This is a chromatographic technique which encompasses the mobile phase region between reversed-phase chromatography (RP) and organic normal phase chromatography (ONP). HILIC is used to achieve unique selectivity for hydrophilic compounds,[35] showing normal phase elution order, using "reversed-phase solvents", i.e., relatively polar mostly non-aqueous solvents in the mobile phase.[34] Many biological molecules, especially those found in biological fluids, are small polar compounds that do not retain well by reversed phase-HPLC. This has made hydrophilic interaction LC (HILIC) an attractive alternative and useful approach for analysis of polar molecules. Additionally, because HILIC is routinely used with traditional aqueous mixtures with polar organic solvents such as ACN and methanol, it can be easily coupled to MS.[35]
Isocratic and gradient elution
[edit]
A separation in which the mobile phase composition remains constant throughout the procedure is termed isocratic (meaning constant composition). The word was coined by Csaba Horvath who was one of the pioneers of HPLC.[36][37]
The mobile phase composition does not have to remain constant. A separation in which the mobile phase composition is changed during the separation process is described as a gradient elution.[38][39] For example, a gradient can start at 10% methanol in water, and end at 90% methanol in water after 20 minutes. The two components of the mobile phase are typically termed "A" and "B"; A is the "weak" solvent which allows the solute to elute only slowly, while B is the "strong" solvent which rapidly elutes the solutes from the column. In reversed-phase chromatography, solvent A is often water or an aqueous buffer, while B is an organic solvent miscible with water, such as acetonitrile, methanol, THF, or isopropanol.
In isocratic elution, peak width increases with retention time linearly according to the equation for N, the number of theoretical plates. This can be a major disadvantage when analyzing a sample that contains analytes with a wide range of retention factors. Using a weaker mobile phase, the runtime is lengthened and results in slowly eluting peaks to be broad, leading to reduced sensitivity. A stronger mobile phase would improve issues of runtime and broadening of later peaks but results in diminished peak separation, especially for quickly eluting analytes which may have insufficient time to fully resolve. This issue is addressed through the changing mobile phase composition of gradient elution.

By starting from a weaker mobile phase and strengthening it during the runtime, gradient elution decreases the retention of the later-eluting components so that they elute faster, giving narrower (and taller) peaks for most components, while also allowing for the adequate separation of earlier-eluting components. This also improves the peak shape for tailed peaks, as the increasing concentration of the organic eluent pushes the tailing part of a peak forward. This also increases the peak height (the peak looks "sharper"), which is important in trace analysis. The gradient program may include sudden "step" increases in the percentage of the organic component, or different slopes at different times – all according to the desire for optimum separation in minimum time.
In isocratic elution, the retention order does not change if the column dimensions (length and inner diameter) change – that is, the peaks elute in the same order. In gradient elution, however, the elution order may change as the dimensions or flow rate change. if they are no scaled down or up according to the change[40]
The driving force in reversed phase chromatography originates in the high order of the water structure. The role of the organic component of the mobile phase is to reduce this high order and thus reduce the retarding strength of the aqueous component.
Parameters
[edit]Theoretical
[edit]The theory of high performance liquid chromatography-HPLC is, at its core, the same as general chromatography theory.[41] This theory has been used as the basis for system-suitability tests, as can be seen in the USP Pharmacopaeia,[42] which are a set of quantitative criteria, which test the suitability of the HPLC system to the required analysis at any step of it.
This relation is also represented as a normalized unit-less factor known as the retention factor, or retention parameter, which is the experimental measurement of the capacity ratio, as shown in the Figure of Performance Criteria as well. tR is the retention time of the specific component and t0 is the time it takes for a non-retained substance to elute through the system without any retention, thus it is called the Void Time.
The ratio between the retention factors, k', of every two adjacent peaks in the chromatogram is used in the evaluation of the degree of separation between them, and is called selectivity factor, α, as shown in the Performance Criteria graph.
The plate count N as a criterion for system efficiency was developed for isocratic conditions, i.e., a constant mobile phase composition throughout the run. In gradient conditions, where the mobile phase changes with time during the chromatographic run, it is more appropriate to use the parameter peak capacity Pc as a measure for the system efficiency.[43] The definition of peak capacity in chromatography is the number of peaks that can be separated within a retention window for a specific pre-defined resolution factor, usually ~1. It could also be envisioned as the runtime measured in number of peaks' average widths. The equation is shown in the Figure of the performance criteria. In this equation tg is the gradient time and w(ave) is the average peaks width at the base.

The parameters are largely derived from two sets of chromatographic theory: plate theory (as part of partition chromatography), and the rate theory of chromatography / Van Deemter equation. Of course, they can be put in practice through analysis of HPLC chromatograms, although rate theory is considered the more accurate theory.
They are analogous to the calculation of retention factor for a paper chromatography separation, but describes how well HPLC separates a mixture into two or more components that are detected as peaks (bands) on a chromatogram. The HPLC parameters are the: efficiency factor(N), the retention factor (kappa prime), and the separation factor (alpha). Together the factors are variables in a resolution equation, which describes how well two components' peaks separated or overlapped each other. These parameters are mostly only used for describing HPLC reversed phase and HPLC normal phase separations, since those separations tend to be more subtle than other HPLC modes (e.g., ion exchange and size exclusion).
Void volume is the amount of space in a column that is occupied by solvent. It is the space within the column that is outside of the column's internal packing material. Void volume is measured on a chromatogram as the first component peak detected, which is usually the solvent that was present in the sample mixture; ideally the sample solvent flows through the column without interacting with the column, but is still detectable as distinct from the HPLC solvent. The void volume is used as a correction factor.
Efficiency factor (N) practically measures how sharp component peaks on the chromatogram are, as ratio of the component peak's area ("retention time") relative to the width of the peaks at their widest point (at the baseline). Peaks that are tall, sharp, and relatively narrow indicate that separation method efficiently removed a component from a mixture; high efficiency. Efficiency is very dependent upon the HPLC column and the HPLC method used. Efficiency factor is synonymous with plate number, and the 'number of theoretical plates'.
Retention factor (kappa prime) measures how long a component of the mixture stuck to the column, measured by the area under the curve of its peak in a chromatogram (since HPLC chromatograms are a function of time). Each chromatogram peak will have its own retention factor (e.g., kappa1 for the retention factor of the first peak). This factor may be corrected for by the void volume of the column.
Separation factor (alpha) is a relative comparison on how well two neighboring components of the mixture were separated (i.e., two neighboring bands on a chromatogram). This factor is defined in terms of a ratio of the retention factors of a pair of neighboring chromatogram peaks, and may also be corrected for by the void volume of the column. The greater the separation factor value is over 1.0, the better the separation, until about 2.0 beyond which an HPLC method is probably not needed for separation. Resolution equations relate the three factors such that high efficiency and separation factors improve the resolution of component peaks in an HPLC separation.
Internal diameter
[edit]
The internal diameter (ID) of an HPLC column is an important parameter.[44] It can influence the detection response when reduced due to the reduced lateral diffusion of the solute band. It can also affect the separation selectivity, when flow rate and injection volumes are not scaled down or up proportionally to the smaller or larger diameter used, both in the isocratic and in gradient modes.[45] It determines the quantity of analyte that can be loaded onto the column. Larger diameter columns are usually seen in preparative applications, such as the purification of a drug product for later use.[46] Low-ID columns have improved sensitivity and lower solvent consumption in the recent ultra-high performance liquid chromatography (UHPLC).[47]
Larger ID columns (over 10 mm) are used to purify usable amounts of material because of their large loading capacity.
Analytical scale columns (4.6 mm) have been the most common type of columns, though narrower columns[47] are rapidly gaining in popularity. They are used in traditional quantitative analysis of samples and often use a UV-Vis absorbance detector.
Narrow-bore columns (1–2 mm) are used for applications when more sensitivity is desired either with special UV-vis detectors, fluorescence detection or with other detection methods like liquid chromatography-mass spectrometry
Capillary columns (under 0.3 mm) are used almost exclusively with alternative detection means such as mass spectrometry. They are usually made from fused silica capillaries, rather than the stainless steel tubing that larger columns employ.
Particle size
[edit]Most traditional HPLC is performed with the stationary phase attached to the outside of small spherical silica particles (very small beads). These particles come in a variety of sizes with 5 μm beads being the most common. Smaller particles generally provide more surface area and better separations, but the pressure required for optimum linear velocity increases by the inverse of the particle diameter squared.[48][49][50]
According to the equations[51] of the column velocity, efficiency and backpressure, reducing the particle diameter by half and keeping the size of the column the same, will double the column velocity and efficiency; but four times increase the backpressure. And the small particles HPLC also can decrease the width broadening.[52] Larger particles are used in preparative HPLC (column diameters 5 cm up to >30 cm) and for non-HPLC applications such as solid-phase extraction.
Pore size
[edit]Many stationary phases are porous to provide greater surface area. Small pores provide greater surface area while larger pore size has better kinetics, especially for larger analytes. For example, a protein which is only slightly smaller than a pore might enter the pore but does not easily leave once inside.
Pump pressure
[edit]Pumps vary in pressure capacity, but their performance is measured on their ability to yield a consistent and reproducible volumetric flow rate. Pressure may reach as high as 60 MPa (6000 lbf/in2), or about 600 atmospheres. Modern HPLC systems have been improved to work at much higher pressures, and therefore are able to use much smaller particle sizes in the columns (<2 μm). These "ultra high performance liquid chromatography" systems or UHPLCs, which could also be known as ultra high pressure chromatography systems,[53] can work at up to 120 MPa (17,405 lbf/in2), or about 1200 atmospheres.[54] The term "UPLC"[55] is a trademark of the Waters Corporation, but is sometimes used to refer to the more general technique of UHPLC.
Detectors
[edit]HPLC detectors fall into two main categories: universal or selective. Universal detectors typically measure a bulk property (e.g., refractive index) by measuring a difference of a physical property between the mobile phase and mobile phase with solute while selective detectors measure a solute property (e.g., UV-Vis absorbance) by simply responding to the physical or chemical property of the solute.[56] HPLC most commonly uses a UV-Vis absorbance detector; however, a wide range of other chromatography detectors can be used. A universal detector that complements UV-Vis absorbance detection is the charged aerosol detector (CAD). A kind of commonly utilized detector includes refractive index detectors, which provide readings by measuring the changes in the refractive index of the eluant as it moves through the flow cell. In certain cases, it is possible to use multiple detectors, for example LCMS normally combines UV-Vis with a mass spectrometer.
When used with an electrochemical detector (ECD) the HPLC-ECD selectively detects neurotransmitters such as: norepinephrine, dopamine, serotonin, glutamate, GABA, acetylcholine and others in neurochemical analysis research applications.[57] The HPLC-ECD detects neurotransmitters to the femtomolar range. Other methods to detect neurotransmitters include liquid chromatography-mass spectrometry, ELISA, or radioimmunoassays.
Autosamplers
[edit]Large numbers of samples can be automatically injected onto an HPLC system, by the use of HPLC autosamplers. In addition, HPLC autosamplers have an injection volume and technique which is exactly the same for each injection, consequently they provide a high degree of injection volume precision. It is possible to enable sample stirring within the sampling-chamber, thus promoting homogeneity.[58]
Applications
[edit]Manufacturing
[edit]HPLC has many applications in both laboratory and clinical science. It is a common technique used in pharmaceutical development, as it is a dependable way to obtain and ensure product purity.[59] While HPLC can produce extremely high quality (pure) products, it is not always the primary method used in the production of bulk drug materials.[60] According to the European pharmacopoeia, HPLC is used in only 15.5% of syntheses.[61] However, it plays a role in 44% of syntheses in the United States pharmacopoeia.[62] This could possibly be due to differences in monetary and time constraints, as HPLC on a large scale can be an expensive technique. An increase in specificity, precision, and accuracy that occurs with HPLC unfortunately corresponds to an increase in cost.
Legal
[edit]This technique is also used for detection of illicit drugs in various samples.[63] The most common method of drug detection has been an immunoassay.[64] This method is much more convenient. However, convenience comes at the cost of specificity and coverage of a wide range of drugs, therefore, HPLC has been used as well as an alternative method. As HPLC is a method of determining (and possibly increasing) purity, using HPLC alone in evaluating concentrations of drugs was somewhat insufficient. Therefore, HPLC in this context is often performed in conjunction with mass spectrometry.[65] Using liquid chromatography-mass spectrometry (LC-MS) instead of gas chromatography-mass spectrometry (GC-MS) circumvents the necessity for derivitizing with acetylating or alkylation agents, which can be a burdensome extra step.[66] LC-MS has been used to detect a variety of agents like doping agents, drug metabolites, glucuronide conjugates, amphetamines, opioids, cocaine, BZDs, ketamine, LSD, cannabis, and pesticides.[67][68] Performing HPLC in conjunction with mass spectrometry reduces the absolute need for standardizing HPLC experimental runs.
Research
[edit]Similar assays can be performed for research purposes, detecting concentrations of potential clinical candidates like anti-fungal and asthma drugs.[69] This technique is obviously useful in observing multiple species in collected samples, as well, but requires the use of standard solutions when information about species identity is sought out. It is used as a method to confirm results of synthesis reactions, as purity is essential in this type of research. However, mass spectrometry is still the more reliable way to identify species.
Medical and health sciences
[edit]Medical use of HPLC typically use mass spectrometer (MS) as the detector, so the technique is called LC-MS[70] or LC-MS/MS for tandem MS, where two types of MS are operated sequentially.[71] When the HPLC instrument is connected to more than one detector, it is called a hyphenated LC system.[citation needed] Pharmaceutical applications[72] are the major users of HPLC, LC-MS and LC-MS/MS.[73] This includes drug development[74] and pharmacology, which is the scientific study of the effects of drugs and chemicals on living organisms,[75] personalized medicine,[76] public health[77][78] and diagnostics.[79] While urine is the most common medium for analyzing drug concentrations, blood serum is the sample collected for most medical analyses with HPLC.[80] One of the most important roles of LC-MS and LC-MS/MS in the clinical lab is the Newborn Screening (NBS) for metabolic disorders[81] and follow-up diagnostics.[82][83] The infants' samples come in the shape of dried blood spot (DBS),[84] which is simple to prepare and transport, enabling safe and accessible diagnostics, both locally and globally.
Other methods of detection of molecules that are useful for clinical studies have been tested against HPLC, namely immunoassays. In one example of this, competitive protein binding assays (CPBA) and HPLC were compared for sensitivity in detection of vitamin D. Useful for diagnosing vitamin D deficiencies in children, it was found that sensitivity and specificity of this CPBA reached only 40% and 60%, respectively, of the capacity of HPLC.[85] While an expensive tool, the accuracy of HPLC is nearly unparalleled.
See also
[edit]- History of chromatography
- Capillary electrochromatography
- Column chromatography
- Csaba Horváth
- Ion chromatography
- Micellar liquid chromatography
References
[edit]- ^ Kazakevich, Yuri; LoBrutto, Rosario, eds. (2007). HPLC for pharmaceutical scientists. Hoboken, NJ: Wiley-Interscience. ISBN 978-0-471-68162-5.
- ^ a b "Chromatography". web.njit.edu. Retrieved 2024-08-05.
- ^ Chaitali Dattatray Harde1, Dr. Amol Navnath Khedkar, Vaishnavi Sanjay Sake. "Review on High Performance Liquid Chromatography" (PDF).
{{cite web}}: CS1 maint: multiple names: authors list (link) CS1 maint: numeric names: authors list (link) - ^ Levin, Shulamit (January 2004). "Reversed Phase Stationary Phases in Pharmaceutical Sciences". Journal of Liquid Chromatography & Related Technologies. 27 (7–9): 1353–1376. doi:10.1081/JLC-120030606. ISSN 1082-6076. S2CID 97490509.
- ^ Gerber, F.; Krummen, M.; Potgeter, H.; Roth, A.; Siffrin, C.; Spoendlin, C. (2004). "Practical aspects of fast reversed-phase high-performance liquid chromatography using 3μm particle packed columns and monolithic columns in pharmaceutical development and production working under current good manufacturing practice". Journal of Chromatography A. 1036 (2): 127–133. doi:10.1016/j.chroma.2004.02.056. PMID 15146913.
- ^ Bayne, Shirley; Carlin, Michelle (2017). Forensic Applications of High Performance Liquid Chromatography (1st ed.). CRC Press. ISBN 9780429251962.
- ^ Seger, Christoph; Salzmann, Linda (2020-08-01). "After another decade: LC–MS/MS became routine in clinical diagnostics". Clinical Biochemistry. Advancement and Applications of Mass Spectrometry in Laboratory Medicine. 82: 2–11. doi:10.1016/j.clinbiochem.2020.03.004. ISSN 0009-9120. PMID 32188572. S2CID 213186669.
- ^ Unger, K. K., ed. (1979-01-01), Chapter 5 Silica columns–packing procedures and performance characteristics, Journal of Chromatography Library, vol. 16, Elsevier, pp. 169–186, doi:10.1016/S0301-4770(08)60809-X, ISBN 978-0-444-41683-4, retrieved 2024-08-05
- ^ Xu, Yan; Cao, Qing; Svec, Frantisek; Fréchet, Jean M.J. (2010-04-15). "Porous polymer monolithic column with surface-bound gold nanoparticles for the capture and separation of cysteine-containing peptides". Analytical Chemistry. 82 (8): 3352–3358. doi:10.1021/ac1002646. ISSN 0003-2700. PMC 2875083. PMID 20302345.
- ^ Panella, Cristina; Ferretti, Rosella; Casulli, Adriano; Cirilli, Roberto (2019-10-01). "Temperature and eluent composition effects on enantiomer separation of carvedilol by high-performance liquid chromatography on immobilized amylose-based chiral stationary phases". Journal of Pharmaceutical Analysis. 9 (5): 324–331. doi:10.1016/j.jpha.2019.04.002. ISSN 2095-1779. PMC 6951491. PMID 31929941.
- ^ "Molecular Interaction of HPLC Stationary Phase". www.imtakt.com. Retrieved 2024-08-08.
- ^ Kadlecová, Zuzana; Kalíková, Květa; Folprechtová, Denisa; Tesařová, Eva; Gilar, Martin (2020-08-16). "Method for evaluation of ionic interactions in liquid chromatography". Journal of Chromatography A. 1625: 461301. doi:10.1016/j.chroma.2020.461301. ISSN 0021-9673. PMID 32709344.
- ^ Dong, Michael (2018). "Ten Common-Sense Corollaries in Pharmaceutical Analysis by High Performance Liquid Chromatography". LCGC Europe. LCGC Europe-08-01-2018. 31 (8): 432–436.
- ^ Snyder, Lloyd R.; Kirkland, Joseph J.; Glajch, Joseph L. (2012). Practical HPLC Method Development (2nd ed.). John Wiley & Sons.
- ^ McMaster, Marvin C. (2007). HPLC: a practical user's guide (2nd ed.). Hoboken, NJ: Wiley-Interscience. ISBN 978-0-471-75401-5.
- ^ Hanai, Toshihiko; Hanai, T. (1999). HPLC: a practical guide. RSC chromatography monographs. Royal Society of Chemistry. Cambridge: Royal Society of Chemistry. ISBN 978-0-85404-515-0.
- ^ a b Karger, Barry L. (1997). "HPLC: Early and Recent Perspectives". Journal of Chemical Education. 74 (1): 45. Bibcode:1997JChEd..74...45K. doi:10.1021/ed074p45.
- ^ a b c d e f Henry, Richard A. (1 February 2009) "The Early Days of HPLC at Dupont" Archived 2020-08-01 at the Wayback Machine. Chromatography Online. Avanstar Communications Inc.
- ^ Giddings, Calvin (1965). Dynamics of Chromatography: Principles and Theory. Marcel Dekker.
- ^ a b Levin, Shulamit (2017). Grinberg, Nelu; Carr, Peter W. (eds.). Solid-Core or Fully Porous Columns in Ultra High-Performance Liquid Chromatography—Which Way to Go for Better Efficiency of the Separation?. Advances in Chromatography. Vol. 55 (1 ed.). Boca Raton: CRC Press. pp. 185–203. ISBN 9781315158075.
- ^ Iler, R.K. (1979) The Chemistry of Silica. John Wiley & Sons. New York.
- ^ Karger, B. L.; Berry, L. V. (1971). "Rapid liquid-chromatographic separation of steroids on columns heavily loaded with stationary phase". Clin. Chem. 17 (8): 757–64. doi:10.1093/clinchem/17.8.757. PMID 4254537.
- ^ Neue, Uwe D. (1997). HPLC columns: theory, technology, and practice. New York, NY: Wiley VCH. ISBN 978-0-471-19037-0.
- ^ Giddings, J. Calvin (1965) Dynamics of Chromatography, Part I. Principles and Theory. Marcel Dekker, Inc., New York. p. 281.
- ^ Ettre, C. (2001). "Milestones in Chromatography: The Birth of Partition Chromatography" (PDF). LCGC. 19 (5): 506–512. Archived from the original (PDF) on 2016-03-04. Retrieved 2016-02-26.
- ^ Martin, A J P; Synge, R L M (1941). "Separation of the higher monoamino-acids by counter-current liquid-liquid extraction: the amino-acid composition of wool". Biochemical Journal. 35 (1–2): 91–121. doi:10.1042/bj0350091. PMC 1265473. PMID 16747393.
- ^ Lindsay, S.; Kealey, D. (1987). High performance liquid chromatography. Wiley. OSTI 7013902. from review Hung, L. B.; Parcher, J. F.; Shores, J. C.; Ward, E. H. (1988). "Theoretical and experimental foundation for surface-coverage programming in gas–solid chromatography with an adsorbable carrier gas". J. Am. Chem. Soc. 110 (11): 1090–1096. doi:10.1021/ac00162a003.
- ^ Displacement Chromatography. Sacheminc.com. Retrieved 2011-06-07. Archived September 15, 2008, at the Wayback Machine
- ^ LoBrutto, Rosario; Kazakevich, Yuri (2007-01-22), Kazakevich, Yuri; LoBrutto, Rosario (eds.), "Reversed-Phase HPLC", HPLC for Pharmaceutical Scientists (1 ed.), Wiley, pp. 139–239, doi:10.1002/9780470087954.ch4, ISBN 978-0-471-68162-5, retrieved 2023-10-10
- ^ Kazakevich, Yuri; LoBrutto, Rosario (2007-01-22), Kazakevich, Yuri; LoBrutto, Rosario (eds.), "Size-Exclusion Chromatography", HPLC for Pharmaceutical Scientists (1 ed.), Wiley, pp. 263–279, doi:10.1002/9780470087954.ch6, ISBN 978-0-471-68162-5, retrieved 2023-10-10
- ^ Mulloy, Barbara; Heath, Alan; Shriver, Zachary; Jameison, Fabian; Al Hakim, Ali; Morris, Tina S.; Szajek, Anita Y. (2014-08-01). "USP compendial methods for analysis of heparin: chromatographic determination of molecular weight distributions for heparin sodium". Analytical and Bioanalytical Chemistry. 406 (20): 4815–4823. doi:10.1007/s00216-014-7940-3. hdl:1721.1/104914. ISSN 1618-2650. PMID 24958344. S2CID 492085.
- ^ Fritz, James S.; Gjerde, Douglas T. (2000-04-25). Ion Chromatography (1 ed.). Wiley. doi:10.1002/9783527613243. ISBN 978-3-527-29914-0.
- ^ Zhang, Chenhua; Rodriguez, Elliott; Bi, Cong; Zheng, Xiwei; Suresh, Doddavenkatana; Suh, Kyungah; Li, Zhao; Elsebaei, Fawzi; Hage, David S. (2018). "High performance affinity chromatography and related separation methods for the analysis of biological and pharmaceutical agents". Analyst. 143 (2): 374–391. Bibcode:2018Ana...143..374Z. doi:10.1039/C7AN01469D. ISSN 1364-5528. PMC 5768458. PMID 29200216.
- ^ a b McCalley, David V. (2017-11-10). "Understanding and manipulating the separation in hydrophilic interaction liquid chromatography". Journal of Chromatography A. 1523: 49–71. doi:10.1016/j.chroma.2017.06.026. ISSN 1873-3778. PMID 28668366.
- ^ a b Buszewski, Bogusław; Noga, Sylwia (2012). "Hydrophilic interaction liquid chromatography (HILIC)—a powerful separation technique". Analytical and Bioanalytical Chemistry. 402 (1): 231–247. doi:10.1007/s00216-011-5308-5. ISSN 1618-2650. PMC 3249561. PMID 21879300.
- ^ Schellinger, Adam P.; Carr, Peter W. (2006). "Isocratic and gradient elution chromatography: A comparison in terms of speed, retention reproducibility and quantitation". Journal of Chromatography A. 1109 (2): 253–266. doi:10.1016/j.chroma.2006.01.047. PMID 16460742. S2CID 26072994.
- ^ Ettre, L. S.; Zlatkis, A., eds. (1979), "Csaba Horváth", Journal of Chromatography Library, 75 years of Chromatography a Historical Dialogue, vol. 17, Elsevier, pp. 151–158, doi:10.1016/s0301-4770(08)60645-4, ISBN 9780444417541, retrieved 2023-10-15
- ^ Snyder, Lloyd R.; Dolan, John W. (2006). High-Performance Gradient Elution: The Practical Application of the Linear-Solvent-Strength Model. Wiley Interscience. ISBN 978-0470055519.
- ^ Schellinger, Adam P.; Carr, Peter W. (2006). "Isocratic and gradient elution chromatography: A comparison in terms of speed, retention reproducibility and quantitation". Journal of Chromatography A. 19th International Symposium on MicroScale Bioseparations. 1109 (2): 253–266. doi:10.1016/j.chroma.2006.01.047. ISSN 0021-9673. PMID 16460742. S2CID 26072994.
- ^ Dolan, John W. (2014). "LC Method Scaling, Part II: Gradient Separations". LCGC North America. 32 (3): 188–193.
- ^ Martin, A. J. P.; Synge, R. L. M. (1941-12-01). "A new form of chromatogram employing two liquid phases". Biochemical Journal. 35 (12): 1358–1368. doi:10.1042/bj0351358. ISSN 0306-3283. PMC 1265645. PMID 16747422.
- ^ https://www.usp.org/sites/default/files/usp/document/harmonization/gen-chapter/harmonization-november-2021-m99380.pdf [bare URL PDF], CHROMATOGRAPH, Stage 4 Harmonization (December 1, 2022)
- ^ Wren, Stephen A. C. (2005-06-15). "Peak capacity in gradient ultra performance liquid chromatography (UPLC)". Journal of Pharmaceutical and Biomedical Analysis. 38 (2): 337–343. doi:10.1016/j.jpba.2004.12.028. ISSN 0731-7085. PMID 15925228.
- ^ Zelenyánszki, Dóra; Felinger, Attila (2020-10-01). "The Impact of Column Hardware on Efficiency in Liquid Chromatography (LC)". LCGC Europe. LCGC Europe-10-01-2020. 33 (10): 498–504.
- ^ Dolan, John (2014). "LC Method Scaling, Part II: Gradient Separations". LCGC North America. LCGC North America-03-01-2014. 32 (3): 188–193.
- ^ Jensen, Ole Elvang; Kidal, Steffen (2006-03-01). "Using Volumetric Flow to Scaleup Chromatographic Processes". BioPharm International. BioPharm International-03-01-2006. 19 (3).
- ^ a b Walter, Thomas H.; Andrews, Richard W. (2014). "Recent innovations in UHPLC columns and instrumentation". Trends in Analytical Chemistry. 63: 14–20. doi:10.1016/j.trac.2014.07.016. ISSN 0165-9936.
- ^ Majors, Ronald E.. (2010-09-07) Fast and Ultrafast HPLC on sub-2 μm Porous Particles — Where Do We Go From Here? – LC-GC Europe. Lcgceurope.com. Retrieved 2011-06-07.
- ^ Xiang, Y.; Liu Y.; Lee M.L. (2006). "Ultrahigh pressure liquid chromatography using elevated temperature". Journal of Chromatography A. 1104 (1–2): 198–202. doi:10.1016/j.chroma.2005.11.118. PMID 16376355.
- ^ Horváth, Cs.; Preiss B.A.; Lipsky S.R. (1967). "Fast liquid chromatography. Investigation of operating parameters and the separation of nucleotides on pellicular ion exchangers". Analytical Chemistry. 39 (12): 1422–1428. doi:10.1021/ac60256a003. PMID 6073805.
- ^ Nguyen, Dao T.-T.; Guillarme, Davy; Rudaz, Serge; Veuthey, Jean-Luc (2006). "Fast analysis in liquid chromatography using small particle size and high pressure". Journal of Separation Science. 29 (12): 1836–1848. doi:10.1002/jssc.200600189. ISSN 1615-9306. PMID 16970187.
- ^ Gritti, Fabrice; Guiochon, Georges (2013). "The van Deemter equation: Assumptions, limits, and adjustment to modern high performance liquid chromatography". Journal of Chromatography A. 1302: 1–13. doi:10.1016/j.chroma.2013.06.032. PMID 23838304.
- ^ Xiang, Yanqiao; Liu, Yansheng; Lee, Milton L. (2006). "Ultrahigh pressure liquid chromatography using elevated temperature". Journal of Chromatography A. 1104 (1–2): 198–202. doi:10.1016/j.chroma.2005.11.118. PMID 16376355.
- ^ 1290 Infinity Quaternary Pump Archived 2015-11-20 at the Wayback Machine. Agilent
- ^ waters. "Trademarks : Waters". www.waters.com.
- ^ K., Robards (1994). Principles and practice of modern chromatographic methods. Haddad, P. R., Jackson, P. E. Amsterdam: Elsevier/Academic Press. ISBN 9780080571782. OCLC 815471219.
- ^ "Electrochemical Detection (ECD) Fundamentals". Amuza Inc.
- ^ Markovitch, Omer; Ottelé, Jim; Veldman, Obe; Otto, Sijbren (2020). "Automated device for continuous stirring while sampling in liquid chromatography systems". Communications Chemistry. 3 (1): 180. Bibcode:2020CmChe...3..180M. doi:10.1038/s42004-020-00427-5. PMC 9814086. PMID 36703458.
- ^ Gerber, Frederic (May 2004). "Practical aspects of fast reversed-phase high-performance liquid chromatography using 3 μm particle packed columns and monolithic columns in pharmaceutical development and production working under current good manufacturing practice". Journal of Chromatography. 1036 (2): 127–33. doi:10.1016/j.chroma.2004.02.056. PMID 15146913.
- ^ Siddiqui, Masoom Raza; AlOthman, Zeid A.; Rahman, Nafisur (2013). "Analytical techniques in pharmaceutical analysis: A review". Arabian Journal of Chemistry. 10: S1409–S1421. doi:10.1016/j.arabjc.2013.04.016.
- ^ The European Pharmacopoeia, 2002. fourth ed., Council of Europe, Strasbourg.
- ^ United States Pharmacopoeia, 2004. 27th ed. The USP Convention Inc., Rockville, MD.
- ^ Merone, Giuseppe M.; Tartaglia, Angela; Rossi, Sandra; Santavenere, Francesco; Bassotti, Elisa; D'Ovidio, Cristian; Bonelli, Martina; Rosato, Enrica; de Grazia, Ugo; Locatelli, Marcello; Savini, Fabio (2021). "Fast Quantitative LC-MS/MS Determination of Illicit Substances in Solid and Liquid Unknown Seized Samples". Analytical Chemistry. 93 (49): 16308–16313. doi:10.1021/acs.analchem.1c03310. ISSN 0003-2700. PMC 8674870. PMID 34843645.
- ^ Pesce, Amadeo; Rosenthal, Murray; West, Robert; West, Cameron; Crews, Bridgit; Mikel, Charles; Almazan, Perla; Latyshev, Sergey (2010-06-01). "An evaluation of the diagnostic accuracy of liquid chromatography-tandem mass spectrometry versus immunoassay drug testing in pain patients". Pain Physician. 13 (3): 273–281. PMID 20495592.
- ^ Tsai, I.-Lin; Weng, Te-I.; Tseng, Yufeng J.; Tan, Happy Kuy-Lok; Sun, Hsiao-Ju; Kuo, Ching-Hua (2013-12-01). "Screening and confirmation of 62 drugs of abuse and metabolites in urine by ultra-high-performance liquid chromatography-quadrupole time-of-flight mass spectrometry". Journal of Analytical Toxicology. 37 (9): 642–651. doi:10.1093/jat/bkt083. PMID 24084874.
- ^ Weinmann, W.; Renz, M.; Vogt, S.; Pollak, S. (2000-01-01). "Automated solid-phase extraction and two-step derivatisation for simultaneous analysis of basic illicit drugs in serum by GC/MS". International Journal of Legal Medicine. 113 (4): 229–235. doi:10.1007/s004149900098. PMID 10929239. S2CID 20451772.
- ^ Kolmonen, Marjo; Leinonen, Antti; Pelander, Anna; Ojanperä, Ilkka (2007-02-28). "A general screening method for doping agents in human urine by solid phase extraction and liquid chromatography/time-of-flight mass spectrometry". Analytica Chimica Acta. 585 (1): 94–102. Bibcode:2007AcAC..585...94K. doi:10.1016/j.aca.2006.12.028. PMID 17386652.
- ^ Pelander, Anna; Ojanperä, Ilkka; Laks, Suvi; Rasanen, Ilpo; Vuori, Erkki (2003-11-01). "Toxicological screening with formula-based metabolite identification by liquid chromatography/time-of-flight mass spectrometry". Analytical Chemistry. 75 (21): 5710–5718. doi:10.1021/ac030162o. PMID 14588010.
- ^ Nobilis, Milan; Pour, Milan; Senel, Petr; Pavlík, Jan; Kunes, Jirí; Voprsalová, Marie; Kolárová, Lenka; Holcapek, Michal (2007-06-15). "Metabolic profiling of a potential antifungal drug, 3-(4-bromophenyl)-5-acetoxymethyl-2,5-dihydrofuran-2-one, in mouse urine using high-performance liquid chromatography with UV photodiode-array and mass spectrometric detection". Journal of Chromatography B. 853 (1–2): 10–19. doi:10.1016/j.jchromb.2007.02.045. PMID 17400036.
- ^ Gu, Jatin; Patel, Kumar; Shah, Dhiren (2016). "APPLICATION OF LC-MS". PharmaTutor.
- ^ Tallam, Anil Kumar; Alapati, Sahithi; Nuli, Mohana Vamsi (2023). "A review on bioanalytical method development and validation of anticancer drugs by using lc/ms/ms and its applications on routine analysis". Journal of Integral Sciences: 4–19. doi:10.37022/jis.v6i1.51. ISSN 2581-5679. S2CID 257295079.
- ^ O'Driscoll, Aimee (2021). "HPLC in Pharmaceutical Applications". Lab Manager.
- ^ Beccaria, Marco; Cabooter, Deirdre (2020). "Current developments in LC-MS for pharmaceutical analysis". Analyst. 145 (4): 1129–1157. Bibcode:2020Ana...145.1129B. doi:10.1039/C9AN02145K. hdl:11392/2479221. ISSN 1364-5528. PMID 31971527. S2CID 210866236.
- ^ Gu, Chunang (Christine); Russell, David; Yehl, Peter (2016). "Application of LCMS in small-molecule drug development". European Pharmaceutical Review. 21 (4): 54–57.
- ^ D'Ovidio, Cristian; Locatelli, Marcello; Perrucci, Miryam; Ciriolo, Luigi; Furton, Kenneth G.; Gazioglu, Isil; Kabir, Abuzar; Merone, Giuseppe Maria; de Grazia, Ugo; Ali, Imran; Catena, Antonio Maria; Treglia, Michele; Marsella, Luigi T.; Savini, Fabio (2023). "LC-MS/MS Application in Pharmacotoxicological Field: Current State and New Applications". Molecules. 28 (5): 2127. doi:10.3390/molecules28052127. ISSN 1420-3049. PMC 10004468. PMID 36903374.
- ^ Zhou, Juntuo; Zhong, Lijun (2022). "Applications of liquid chromatography-mass spectrometry based metabolomics in predictive and personalized medicine". Frontiers in Molecular Biosciences. 9. doi:10.3389/fmolb.2022.1049016. ISSN 2296-889X. PMC 9669074. PMID 36406271.
- ^ Mathias, Patricia I.; Connor, Thomas H.; B'Hymer, Clayton (2017). "A review of high performance liquid chromatographic-mass spectrometric urinary methods for anticancer drug exposure of health care workers". Journal of Chromatography B. 1060: 316–324. doi:10.1016/j.jchromb.2017.06.028. ISSN 1570-0232. PMC 5585056. PMID 28654869.
- ^ Hernández, Félix; Sancho, Juan V.; Ibáñez, María; Guerrero, Carlos (2007). "Antibiotic residue determination in environmental waters by LC-MS". TrAC Trends in Analytical Chemistry. Pharmaceutical-residue analysis. 26 (6): 466–485. doi:10.1016/j.trac.2007.01.012. ISSN 0165-9936.
- ^ Seger, Christoph; Salzmann, Linda (2020). "After another decade: LC–MS/MS became routine in clinical diagnostics". Clinical Biochemistry. Advancement and Applications of Mass Spectrometry in Laboratory Medicine. 82: 2–11. doi:10.1016/j.clinbiochem.2020.03.004. ISSN 0009-9120. PMID 32188572. S2CID 213186669.
- ^ Sundström, Mira; Pelander, Anna; Angerer, Verena; Hutter, Melanie; Kneisel, Stefan; Ojanperä, Ilkka (2013-10-01). "A high-sensitivity ultra-high performance liquid chromatography/high-resolution time-of-flight mass spectrometry (UHPLC-HR-TOFMS) method for screening synthetic cannabinoids and other drugs of abuse in urine". Analytical and Bioanalytical Chemistry. 405 (26): 8463–8474. doi:10.1007/s00216-013-7272-8. PMID 23954996. S2CID 25743579.
- ^ Gelb, Michael H.; Basheeruddin, Khaja; Burlina, Alberto; Chen, Hsiao-Jan; Chien, Yin-Hsiu; Dizikes, George; Dorley, Christine; Giugliani, Roberto; Hietala, Amy; Hong, Xinying; Kao, Shu-Min; Khaledi, Hamid; Klug, Tracy; Kubaski, Francyne; Liao, Hsuan-Chieh (2022). "Liquid Chromatography–Tandem Mass Spectrometry in Newborn Screening Laboratories". International Journal of Neonatal Screening. 8 (4): 62. doi:10.3390/ijns8040062. ISSN 2409-515X. PMC 9781967. PMID 36547379.
- ^ Wang, Yanyun; Sun, Yun; Jiang, Tao (2019). "Clinical Application of LC–MS/MS in the Follow-Up for Treatment of Children with Methylmalonic Aciduria". Advances in Therapy. 36 (6): 1304–1313. doi:10.1007/s12325-019-00955-0. ISSN 1865-8652. PMID 31049874. S2CID 143432183.
- ^ Arunkumar, Nivethitha; Langan, Thomas J.; Stapleton, Molly; Kubaski, Francyne; Mason, Robert W.; Singh, Rajendra; Kobayashi, Hironori; Yamaguchi, Seiji; Suzuki, Yasuyuki; Orii, Kenji; Orii, Tadao; Fukao, Toshiyuki; Tomatsu, Shunji (2020). "Newborn screening of mucopolysaccharidoses: past, present, and future". Journal of Human Genetics. 65 (7): 557–567. doi:10.1038/s10038-020-0744-8. ISSN 1435-232X. PMID 32277174. S2CID 92042115.
- ^ Skogvold, Hanne Bendiksen; Rootwelt, Helge; Reubsaet, Léon; Elgstøen, Katja Benedikte Prestø; Wilson, Steven Ray (2023). "Dried blood spot analysis with liquid chromatography and mass spectrometry: Trends in clinical chemistry". Journal of Separation Science. 46 (15): e2300210. doi:10.1002/jssc.202300210. hdl:10852/105845. ISSN 1615-9306. PMID 37269205. S2CID 259047202.
- ^ Zahedi Rad, Maliheh; Neyestani, Tirang Reza; Nikooyeh, Bahareh; Shariatzadeh, Nastaran; Kalayi, Ali; Khalaji, Niloufar; Gharavi, Azam (2015-01-01). "Competitive Protein-binding assay-based Enzyme-immunoassay Method, Compared to High-pressure Liquid Chromatography, Has a Very Lower Diagnostic Value to Detect Vitamin D Deficiency in 9–12 Years Children". International Journal of Preventive Medicine. 6: 67. doi:10.4103/2008-7802.161069. PMC 4542329. PMID 26330983.
Further reading
[edit]- L. R. Snyder, J.J. Kirkland, and J. W. Dolan, Introduction to Modern Liquid Chromatography, John Wiley & Sons, New York, 2009.
- M.W. Dong, Modern HPLC for practicing scientists. Wiley, 2006.
- L. R. Snyder, J.J. Kirkland, and J. L. Glajch, Practical HPLC Method Development, John Wiley & Sons, New York, 1997.
- S. Ahuja and H. T. Rasmussen (ed), HPLC Method Development for Pharmaceuticals, Academic Press, 2007.
- S. Ahuja and M.W. Dong (ed), Handbook of Pharmaceutical Analysis by HPLC, Elsevier/Academic Press, 2005.
- Y. V. Kazakevich and R. LoBrutto (ed.), HPLC for Pharmaceutical Scientists, Wiley, 2007.
- U. D. Neue, HPLC Columns: Theory, Technology, and Practice, Wiley-VCH, New York, 1997.
- M. C. McMaster, HPLC, a practical user's guide, Wiley, 2007.
