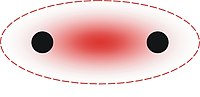
Intramolecular force
An intramolecular force is any force that holds together the atoms making up a molecule or compound.[1] They contain all types of chemical bond. They are stronger than intermolecular forces, which are present between atoms or molecules that are not actually bonded.
Types of intramolecular force
There are several types of intramolecular forces, distinguished by the types of constituent atoms and the behavior of electrons:
- Ionic, which generally form between a metal and nonmetal, such as sodium and chlorine in NaCl. Electrons in an ionic bond tend to mostly be found around one of the two constituent atoms due to the large electronegativity difference between the two atoms; this is often described as one atom giving electrons to the other. In the case of NaCl, sodium would give an electron to chlorine.
- Covalent, which generally form between two nonmetals. Examples include nitrogen dioxide. Electrons in a covalent bond are essentially shared between the constituent atoms. There are several types of covalent bond: in polar covalent bonds, electrons are more likely to be found around one of the two atoms, whereas in nonpolar covalent bonds, electrons are evenly shared. Homonuclear diatomic molecules are pure covalent. The polarity of a covalent bond is determined by the electronegativities of each atom, and a polar covalent bond usually creates a dipole moment.
- Metallic, which generally forms within a pure metal or metal alloy. Metallic electrons are generally delocalized; the result is a large number of free electrons around positive nuclei, sometimes called an electron sea.
They differ in the magnitude of their bond enthalpies, and thus affect the physical and chemical properties of compounds in different ways.
See also
References
- ^ Zumdahl, Stephen S., & Zumdahl, Susan A. Chemistry. Houghton Mifflin, 2007, ISBN 0618713700