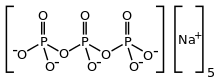
Sodium triphosphate
| |
| Names | |
|---|---|
| IUPAC name
Pentasodium triphosphate
| |
| Other names
sodium tripolyphosphate, polygon, STPP
| |
| Identifiers | |
| ECHA InfoCard | 100.028.944 |
| E number | E451 (thickeners, ...) |
PubChem CID
|
|
| RTECS number |
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| Properties | |
| Na5P3O10 | |
| Molar mass | 367.864 g/mol |
| Appearance | white powder |
| Density | 2.52 g/cm3 |
| Melting point | 622 °C (1,152 °F; 895 K) |
| 14.5 g/100 mL (25 °C) | |
| Hazards | |
| NFPA 704 (fire diamond) | |
| Flash point | Non-flammable |
| Safety data sheet (SDS) | ICSC 1469 |
| Related compounds | |
Other anions
|
Trisodium phosphate Tetrasodium pyrophosphate Sodium hexametaphosphate |
Other cations
|
Pentapotassium triphosphate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Sodium triphosphate (STP), also sodium tripolyphosphate (STPP), or tripolyphosphate (TPP),[1]) is an inorganic compound with formula Na5P3O10. It is the sodium salt of the polyphosphate penta-anion, which is the conjugate base of triphosphoric acid. It is produced on a large scale as a component of many domestic and industrial products, especially detergents. Environmental problems associated with eutrophication are attributed to its widespread use.[2]
Preparation and properties
[edit]Sodium tripolyphosphate is produced by heating a stoichiometric mixture of disodium phosphate, Na2HPO4, and monosodium phosphate, NaH2PO4, under carefully controlled conditions.[2]
- 2 Na2HPO4 + NaH2PO4 → Na5P3O10 + 2 H2O
In this way, approximately 2 million tons are produced annually.[3]
STPP is a colourless salt, which exists both in anhydrous form and as the hexahydrate. The anion can be described as the pentanionic chain [O3POP(O)2OPO3]5−.[4][5] Many related di-, tri-, and polyphosphates are known including the cyclic triphosphate (e.g. sodium trimetaphosphate). It binds strongly to metal cations as both a bidentate and tridentate chelating agent.

Uses
[edit]Detergents
[edit]The majority of STPP is consumed as a component of commercial detergents. It serves as a "builder", industrial jargon for a water softener. In hard water (water that contains high concentrations of Mg2+ and Ca2+), detergents are deactivated. Being a highly charged chelating agent, TPP5− binds to dications tightly and prevents them from interfering with the sulfonate detergent.[3]
Food
[edit]STPP is a preservative for seafood, meats, poultry, and animal feeds.[3] It is common in food production as E number E451. In foods, STPP is used as an emulsifier and to retain moisture. Many governments regulate the quantities allowed in foods, as it can substantially increase the sale weight of seafood in particular. The United States Food and Drug Administration lists STPP as Generally recognized as safe.[6]
Other
[edit]Other uses (hundreds of thousands of tons/year) include ceramics (decrease the viscosity of glazes up to a certain limit), leather tanning (as masking agent and synthetic tanning agent - SYNTAN), anticaking agents, setting retarders, flame retardants, paper, anticorrosion pigments, textiles, rubber manufacture, fermentation, antifreeze."[3] TPP is used as a polyanion crosslinker in polysaccharide based drug delivery.[7] Toothpaste may contain sodium triphosphate.[8][9][10][11][12][13][14]
Health effects
[edit]High serum phosphate concentration has been identified as a predictor of cardiovascular events and mortality. Whilst phosphate is present in the body and food in organic forms, inorganic forms of phosphate such as sodium triphosphate are readily adsorbed and can result in elevated phosphate levels in serum.[15] Salts of polyphosphate anions are moderately irritating to skin and mucous membranes because they are mildly alkaline.[1]
Environmental effects
[edit]Because it is very water-soluble, STPP is not significantly removed by waste water treatment. STPP hydrolyses to phosphate, which is assimilated into the natural phosphorus cycle. Detergents containing phosphorus contribute to the eutrophication of many fresh waters.[1]

See also
[edit]- Sodium trimetaphosphate, a cyclic triphosphate
References
[edit]- ^ a b c Complexing agents, Environmental and Health Assessment of Substances in Household Detergents and Cosmetic Detergent Products, Danish Environmental Protection Agency Archived 2017-08-24 at the Wayback Machine, Accessed 2008-07-15
- ^ a b Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- ^ a b c d Schrödter, Klaus; Bettermann, Gerhard; Staffel, Thomas; Wahl, Friedrich; Klein, Thomas; Hofmann, Thomas (2008). "Phosphoric Acid and Phosphates". Ullmann's Encyclopedia of Industrial Chemistry. doi:10.1002/14356007.a19_465.pub3. ISBN 978-3527306732. S2CID 94458523.
- ^ Corbridge, D. E. C. (1 March 1960). "The crystal structure of sodium triphosphate, Na5P3O10, phase I". Acta Crystallographica. 13 (3): 263–269. Bibcode:1960AcCry..13..263C. doi:10.1107/S0365110X60000583.
- ^ Davies, D. R.; Corbridge, D. E. C. (1 May 1958). "The crystal structure of sodium triphosphate, Na5P3O10, phase II". Acta Crystallographica. 11 (5): 315–319. Bibcode:1958AcCry..11..315D. doi:10.1107/S0365110X58000876.
- ^ "Substances Added to Food (Formerly EAFUS)".
- ^ Calvo, P.; Remuñán-López, C.; Vila-Jato, J. L.; Alonso, M. J. (3 January 1997). "Novel hydrophilic chitosan-polyethylene oxide nanoparticles as protein carriers". Journal of Applied Polymer Science. 63 (1): 125–132. doi:10.1002/(SICI)1097-4628(19970103)63:1<125::AID-APP13>3.0.CO;2-4.
- ^ Saxton, C. A.; Ouderaa, F. J. G. (January 1989). "The effect of a dentifrice containing zinc citrate and Triclosan on developing gingivitis". Journal of Periodontal Research. 24 (1): 75–80. doi:10.1111/j.1600-0765.1989.tb00860.x. PMID 2524573.
- ^ Lobene, RR; Weatherford, T; Ross, NM; Lamm, RA; Menaker, L (1986). "A modified gingival index for use in clinical trials". Clinical Preventive Dentistry. 8 (1): 3–6. PMID 3485495.
- ^ Lobene, RR; Soparkar, PM; Newman, MB (1982). "Use of dental floss. Effect on plaque and gingivitis". Clinical Preventive Dentistry. 4 (1): 5–8. PMID 6980082.
- ^ Mankodi, Suru; Bartizek, Robert D.; Leslie Winston, J.; Biesbrock, Aaron R.; McClanahan, Stephen F.; He, Tao (January 2005). "Anti-gingivitis efficacy of a stabilized 0.454% stannous fluoride/sodium hexametaphosphate dentifrice. A controlled 6-month clinical trial". Journal of Clinical Periodontology. 32 (1): 75–80. doi:10.1111/j.1600-051X.2004.00639.x. PMID 15642062.
- ^ Mankodi, S; Petrone, DM; Battista, G; Petrone, ME; Chaknis, P; DeVizio, W; Volpe, AR; Proskin, HM (1997). "Clinical efficacy of an optimized stannous fluoride dentifrice, Part 2: A 6-month plaque/gingivitis clinical study, northeast USA". Compendium of Continuing Education in Dentistry. 18 Spec No: 10–5. PMID 12206029.
- ^ Mallatt, Mark; Mankodi, Suru; Bauroth, Karen; Bsoul, Samer A.; Bartizek, Robert D.; He, Tao (September 2007). "A controlled 6-month clinical trial to study the effects of a stannous fluoride dentifrice on gingivitis". Journal of Clinical Periodontology. 34 (9): 762–767. doi:10.1111/j.1600-051X.2007.01109.x. PMID 17645550.
- ^ Lang, Niklaus P. (1990). "Epidemiology of periodontal disease". Archives of Oral Biology. 35: S9–S14. doi:10.1016/0003-9969(90)90125-t. PMID 2088238.
- ^ Ritz, Eberhard; Hahn, Kai; Ketteler, Markus; Kuhlmann, Martin K; Mann, Johannes (2012). "Phosphate Additives in Food—a Health Risk". Deutsches Ärzteblatt International. 109 (4): 49–55. doi:10.3238/arztebl.2012.0049. PMC 3278747. PMID 22334826.

