Terbium compounds
Terbium compounds are compounds formed by the lanthanide metal terbium (Tb). Terbium generally exhibits the +3 oxidation state in these compounds, such as in TbCl3, Tb(NO3)3 and Tb(CH3COO)3. Compounds with terbium in the +4 oxidation state are also known, such as TbO2 and BaTbF6.[1] Terbium can also form compounds in the 0,[2][3] +1[4] and +2 oxidation states.
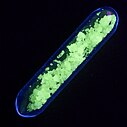
The trivalent terbium ion (Tb3+) is generally colorless in aqueous solution, and when it is irradiated by certain wavelengths of ultraviolet light (such as 254 nm or 365 nm) in solution or crystal form, it will emit green fluorescence. This property has given rise to applications in fields such as optics.[5] The tetravalent terbium ion (Tb4+) is non-luminescent and its coexistence with Tb3+ will reduce the green emission of Tb3+.[6]
Properties of terbium compounds
[edit]| Formula | appearance | symmetry | space group | No | a (nm) | b (nm) | c (nm) | density, g/cm3 |
|---|---|---|---|---|---|---|---|---|
| TbBr3 | white powder (hexahydrate)[7] | trigonal | R3 | 148 | 4.62[8] | |||
| TbCl3 | white powder | hexagonal | P63/m | 176 | 4.35 | |||
| TbF3 | white solid | hexagonal[9] | 7.035[9] | 6.875[9] | 7.23[10] | |||
| Tb(OH)3 | white solid[11] | |||||||
| TbI3 | hydroscopic crystals | trigonal[12][13] | R3 | 148 | 5.2 | |||
| Tb(NO3)3 | colourless crystals (hexahydrate)[14] | monoclinic[15] | P21/c | 14 | 1.2870 | 1.6590 | 2.8723 | |
| Tb2O3 | white crystals | cubic | Ia3[16] | 206[16] | 1.057 | 7.91 | ||
| TbO2 | dark-coloured[17] | cubic | ||||||
| TbP | black crystals | cubic | Fm3m | 225 | 0.56402 | 6.82 | ||
| Tb4O7 | dark brown-black solid | orthorhombic | R3[18] | 148 | 6.5082 | 7.3 | ||
| Tb11O20 | solid | triclinic | P1 | 2 | 6.50992 | 9.8298 | 6.4878 | |
| TbAsO4 | solid | orthorhombic[19] | Fddd | 70 | 7.09 | 6.32 | ||
| TbAsO4 | solid | tetragonal[19] | I41/amd | 141 | 10.081 | 9.957 | 6.321 | |
| TbSbO4 | green (under UV light) | monoclinic[20] | P21/m | 11 | ||||
| TbSe | yellow-red solid[21] | cubic | Fm3m[22] | 225 |
Chalcogenides
[edit]Oxides
[edit]
Terbium has a variety of oxides. The most easily obtained is terbium(III,IV) oxide, which can be produced by the decomposition of terbium compounds such as the hydroxide,[23] the oxalate[24] and the p-aminobenzoate.[25] Terbium(III,IV) oxide, because the oxide contains both trivalent terbium and tetravalent terbium, can be produced by reacting with nitric acid to produce terbium nitrate, releasing oxygen in the progress:[23]
- 2 Tb4O7 + 24 HNO3 → 8 Tb(NO3)3 + 12 H2O + O2↑
It is refluxed in a mixture of acetic acid and hydrochloric acid, which can separate trivalent and tetravalent terbium:[26]
- Tb4O7 + 6 HCl → 2 TbO2 + 2 TbCl3 + 3 H2O
It reacts with dicyandiamide at a high temperature to obtain Tb2O2CN2.[27]
Another common oxide of terbium is terbium(III) oxide, which can be obtained from the reduction of hydrogen from terbium(III,IV) oxide at 1300 °C.[28] A p-type semiconductor is formed after doping with calcium.[29]
Terbium(IV) oxide can be prepared by treating terbium(III,IV) oxide with dilute hydrochloric acid,[30] its hydrate TbO2·xH2O can be obtained by oxidizing terbium(III) hydroxide with potassium persulfate in the presence of silver nitrate.[31] Terbium(IV) oxide can form mixed crystals with praseodymium(IV) oxide.[32]
Other chalcogenides
[edit]Terbium(III) sulfide is one of the sulfides of terbium, which can be obtained by reacting with sulfur in a stoichiometric ratio.[33] It can also be obtained by reacting terbium(III,IV) oxide with carbon disulfide and hydrogen sulfide at high temperature.[34] It reacts with hydrofluoric acid solution to give terbium(III) fluoride hemihydrate.[34] Terbium(III) selenide can be obtained by the reaction of terbium polyselenide TbSe1.9 with metal terbium, which can form black needle-like crystals with U2S3 structure and space group Pnma.[35] Terbium monochalcogenides, TbZ (Z = S, Se or Te), can be prepared by directly reacting terbium with the corresponding chalcogen. These chalcogenides are black and have a NaCl structure. They have metallic conductivity and consist of Ln3+ and Z2- ions with 1 electron from each cation delocalized in a conduction band.[17]
Halides and halogen complexes
[edit]
Terbium can form four trihalides in the form TbX3 (X=F, Cl, Br, I), which, except the fluoride, are easily soluble in water, and are strong electrolytes in water. They can be prepared by reacting terbium with the corresponding halogen:[36]
- 2Tb (s) + 3F2 (g) → 2TbF3 (s) [a white substance]
- 2Tb (s) + 3Cl2 (g) → 2TbCl3 (s) [a white substance]
- 2Tb (s) + 3Br2 (g) → 2TbBr3 (s) [a white substance]
- 2Tb (s) + 3I2 (g) → 2TbI3 (s)
Anhydrous terbium halides can be prepared by reacting oxides or halides hydrates:[37]
- Tb2O3 + 6 NH4Cl → 2 TbCl3 + 3 H2O + 6 NH3↑
- TbCl3·6H2O + 6 SOCl2 → TbCl3 + 6 SO2↑ + 12 HCl↑
Terbium(II) halides are obtained by annealing Tb(III) halides in presence of metallic Tb in tantalum containers. Terbium also forms a sesquichloride Tb2Cl3, which can be further reduced to TbCl by annealing at 800 °C. This terbium(I) chloride forms platelets with layered graphite-like structure.[38]
Terbium(IV) fluoride is the only halide that tetravalent terbium can form, and has strong oxidizing properties. It is also a strong fluorinating agent, emitting relatively pure atomic fluorine when heated, rather than the mixture of fluoride vapors emitted from cobalt(III) fluoride or cerium(IV) fluoride.[39] It can be obtained by reacting terbium(III) chloride or terbium(III) fluoride with fluorine gas at 320 °C:[40]
- 2 TbF3 + F2 → 2 TbF4
When TbF4 and CsF is mixed in a stoichiometric ratio, in a fluorine gas atmosphere, CsTbF5 is obtained. It is an orthorhombic crystal, with space group Cmca, with a layered structure composed of [TbF8]4− and 11-coordinated Cs+.[41] The compound BaTbF6 can be prepared in a similar method. It is an orthorhombic crystal, with space group Cmma. The compound [TbF8]4− also exists.[42]
Organoterbium compounds
[edit]Organoterbium compounds are a class of organic metal compounds containing Tb-C bonds. The cyclopentadienyl complexes of terbium were studied in the early stage. They can be prepared by the reaction of sodium cyclopentadienide and anhydrous terbium halide in tetrahydrofuran, such as:[43]
- TbCl3 + 3 C5H5Na → (C5H5)3Tb + 3 NaCl
- TbI2 + 2 (C5HiPr4)Na → (C5HiPr4)2Tb + 2 NaI
However, this compound has limited usage and academic interest.[44]
Like the other lanthanides, metal-carbon σ bonds are found in alkyls of terbium such as [TbMe6]3− and Tb[CH(SiMe3)2]3.[44] The alkyls and aryls can be prepared by metathesis in tetrahydrofuran on ether solutions:[17]
- TbCl3 + 3 LiR → TbR3 + 3 LiCl
- TbCl3 + 4 LiR → Li[TbR4] + 3 LiCl3
Other compounds
[edit]Oxoacid salts
[edit]Terbium(III) sulfate can be obtained by the reaction of terbium(III,IV) oxide and concentrated sulfuric acid. It can crystallize colorless octahydrate crystals in water, which is isostructural with the corresponding praseodymium compound.[45] The anhydrate can be obtained by heating the octahydrate, and an exothermic reaction occurs when the anhydrate is rehydrated.[46] Terbium(III) hydroxide can be obtained by reacting terbium with water.[36] It reacts with acids to produce terbium(III) salts. It decomposes to TbO(OH) at an elevated temperature, and upon further heating, will decompose to terbium(III) oxide.[11]
Terbium(III) nitrate can be obtained by reacting terbium(III) oxide with nitric acid and crystallizing. The crystals are dried with 45~55% sulfuric acid to obtain the hexahydrate.[47] The basic salt TbONO3 can be obtained by heating the hydrate, and its anhydrate can be obtained by the reaction of terbium(III) oxide and dinitrogen tetroxide.[48] Terbium(III) phosphate can be obtained by the reaction of diammonium hydrogen phosphate and terbium(III) nitrate, and the reaction produces a hexagonal monohydrate, which can emit the characteristic green light of terbium (543 nm) under the excitation of 355 nm wavelength.[49] It can also be obtained by the reaction of sodium phosphate and terbium(III) chloride in solution, and the precipitated dihydrate is calcined at 800 °C to obtain the anhydrous form.[50] Terbium(III) arsenate is an orthorhombic crystal at 77 K with space group Fddd, and undergoes a phase transition at 27.7 K to form a tetragonal crystal with space group I41/amd,[19] which is a ferromagnet below 1.5 K.[19] It can be produced by reacting sodium arsenate and terbium(III) chloride.[51] Terbium(III) antimonate (TbSbO4) is a monoclinic crystal with space group P21/m (No. 11).[20]
Terbium(III) carbonate can be obtained by reacting terbium(III) chloride with saturated carbon dioxide solution in sodium bicarbonate, and the product also needs to be washed with water saturated with carbon dioxide.[52] The germanates TbIII13(GeO4)6O7(OH) and K2TbIVGe2O7 can be synthesized at high temperature and pressure, and they are colorless crystals of trigonal and monoclinic systems, respectively.[53] The tetrahydrate of terbium(III) acetate can lose hydration at 60 °C, obtaining the anhydrate at 180 °C, which starts to decompose at 220 °C, forming terbium oxide at 650 °C.[54]
Terbium borate can be obtained by reacting terbium oxide with boric acid:[55]
- 2 Tb4O7 + 8 H3BO3 → 8 TbBO3 + 12 H2O + O2↑
The single crystal of its hexagonal phase can be obtained by the Czochralski method; it can also form a solid of the triclinic system, which can be obtained by the sol-gel method.[56] The composite borates TbFe3(BO3)4 and TbAl3(BO3)4 can also be obtained by a similar method.[57][58] Terbium(III) oxide, terbium(III) chloride and boron trioxide react in a caesium chloride solution to obtain terbium oxychloride borate Tb4O4Cl(BO3), which is a monoclinic crystal with space group P21/n.[59] Both aluminate Tb3Al5O12[60] and gallate Tb3Ga5O12[61][62] can be used as magneto-optical materials.
Pnictides
[edit]All the terbium pnictides form crystals of the cubic crystal system, with the space group of Fm3m.[63][64][65] Terbium phosphide can be obtained by reacting sodium phosphide and anhydrous terbium(III) chloride at 700 to 800˚C.[66] It undergoes a phase transition at 40 GPa from a NaCl-structure to a CsCl-structure.[67] It can be sintered with zinc sulfide to make a green phosphor layer.[68]
Alloys
[edit]
Terfenol-D
[edit]Terfenol-D is an alloy of terbium, iron and dysprosium, with the formula of TbxDy1−xFe2. It was initially developed in the 1970s by the Naval Ordnance Laboratory in the United States. The technology for manufacturing the material efficiently was developed in the 1980s at Ames Laboratory under a U.S. Navy-funded program.[69] It has the highest magnetostriction of any alloy, with up to 0.002 m/m at saturation.[70] It possess nearly zero magnetocrystalline anisotropy and so exhibits very large magnetostriction at low magnetic fields.[71] Terfenol-D is mostly used for its magnetostrictive properties, in which it changes shape when exposed to magnetic fields in a process called magnetization. Magnetic heat treatment is shown to improve the magnetostrictive properties of Terfenol-D at low compressive stress for certain ratios of Tb and Dy.[72]
Victorium
[edit]Victorium[73] (also called monium, meaning "alone", because its spectral lines stood alone at the end of the ultraviolet spectrum[74]) is an alloy of gadolinium and terbium, which was misidentified as a chemical element in 1898 by the English chemist William Crookes. He identified the new substance, based on an analysis of the unique phosphorescence and other ultraviolet-visible spectral phenomena, as a new chemical element. However, in 1905, French chemist Georges Urbain had proven that to be false, and in fact, a impurity of gadolinium and terbium.[75]
Applications
[edit]Terbium compounds do not have many applications. However, compounds of trivalent terbium can emit green light under excitation, such as terbium(III) oxide which can be used in cathode-ray tube televisions.[76] Terbium compounds are also used in optics due to this property.[5] In addition, terbium compounds have other applications. For example, TbFe2-based compounds can be used as magnetostrictive materials,[77] dielectric Tb3Ga5O12 and Tb3Al5O12 can be used as magneto-optical materials,[60][61][62] terbium(III) fluoride is used for the production of fluoride glasses and electroluminescent thin films and luminescent zinc sulfide[78] and terbium gatifloxacin can be used as drugs.[79] Terbium phosphide is a semiconductor used in high power, high frequency applications and in laser diodes and other photo diodes.[80] CePO4:Tb (Cerium phosphate doped terbium) has potential application in biological imaging and cellular labeling.[81]
Alloys containing terbium are used in the production of electronic devices, mostly as a component of Terfenol-D. The alloy is used in actuators,[82] in sensors, in the SoundBug device (its first commercial application), hydraulic valve drivers[83] and other magnetomechanical devices. It is also used in naval sonar systems. Its strain is also larger than that of another normally used material (PZT8), which allows Terfenol-D transducers to reach greater depths for ocean explorations than past transducers.[84] Its low Young's Modulus brings some complications due to compression at large depths, which are overcome in transducer designs that may reach 1000 ft in depth and only lose a small amount of accuracy of around 1 dB.[83] Due to its high temperature range, Terfenol-D is also useful in deep hole acoustic transducers where the environment may reach high pressure and temperatures like oil holes. Terfenol-D may also be used for hydraulic valve drivers due to its high strain and high force properties.[83] Similarly, magnetostrictive actuators have also been considered for use in fuel injectors for diesel engines because of the high stresses that can be produced.[85]
Pictures of terbium compounds
[edit]See also
[edit]- Europium compounds
- Berkelium compounds (the actinide analogue of terbium)
References
[edit]- ^ 无机化学丛书. pp 187-188. 1.2.3 氧化态及电极电势
- ^ Cloke, F. Geoffrey N. (1993). "Zero Oxidation State Compounds of Scandium, Yttrium, and the Lanthanides". Chem. Soc. Rev. 22: 17–24. doi:10.1039/CS9932200017.
- ^ Arnold, Polly L.; Petrukhina, Marina A.; Bochenkov, Vladimir E.; Shabatina, Tatyana I.; Zagorskii, Vyacheslav V.; Cloke (2003-12-15). "Arene complexation of Sm, Eu, Tm and Yb atoms: a variable temperature spectroscopic investigation". Journal of Organometallic Chemistry. 688 (1–2): 49–55. doi:10.1016/j.jorganchem.2003.08.028.
- ^ Li, Wan-Lu; Chen, Teng-Teng; Chen, Wei-Jia; Li, Jun; Wang, Lai-Sheng (2021). "Monovalent lanthanide(I) in borozene complexes". Nature Communications. 12 (1): 6467. Bibcode:2021NatCo..12.6467L. doi:10.1038/s41467-021-26785-9. PMC 8578558. PMID 34753931.
- ^ a b Tieli, Z; Huichun, Z; Linpei, J (June 1999). "Photochemical fluorescence enhancement of the terbium–lomefloxacin complex and its application". Talanta. 49 (1): 77–82. doi:10.1016/s0039-9140(98)00364-6. PMID 18967577.
- ^ Ueda, Kazushige; Shimizu, Yuhei; Nagamizu, Kouta; Matsuo, Masashi; Honma, Tetsuo (2017). "Luminescence and Valence of Tb Ions in Alkaline Earth Stannates and Zirconates Examined by X-ray Absorption Fine Structures". Inorg. Chem. 56 (20): 12625–12630. doi:10.1021/acs.inorgchem.7b02165.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Brown, D.; Fletcher, S.; Holah, D. G. (1968). "The preparation and crystallographic properties of certain lanthanide and actinide tribromides and tribromide hexahydrates". Journal of the Chemical Society A: Inorganic, Physical, Theoretical: 1889. doi:10.1039/j19680001889.
- ^ americanelements.com: Terbium Bromide
- ^ a b c 无机化学丛书 第七卷 钪 稀土元素. 科学出版社. pp 212. 表 22.24 无水卤化物的物理常数.
- ^ americanelements.com: Terbium Fluoride
- ^ a b 《无机化学丛书》. 第七卷 钪 稀土元素. 易宪武 等主编. 科学出版社. P168~171. (2)氢氧化物[verification needed]
- ^ Wells, A. F. (1984). Structural Inorganic Chemistry (5th ed.). Oxford University Press. p. 421. ISBN 978-0-19-965763-6.
- ^ Asprey, L. B.; Keenan, T. K.; Kruse, F. H. (1964). "Preparation and Crystal Data for Lanthanide and Actinide Triiodides". Inorg. Chem. 3 (8): 1137–1141. doi:10.1021/ic50018a015.
- ^ Moret, Emmanuel; Bünzli, Jean-Claude G.; Schenk, Kurt J. (December 1990). "Structural and luminescence study of europium and terbium nitrate hexahydrates". Inorganica Chimica Acta. 178 (1): 83–88. doi:10.1016/S0020-1693(00)88138-4.
- ^ Tang, Yu; Tang, Kuan Zhen; Zhang, Jian; Tan, Min Yu; Liu, Wei Sheng; Sun, Yu Xi (2006). "酰胺型三足配体硝酸铽超分子配合物的晶体结构与荧光性质" [Crystal structure and luminescent property of the terbium nitrate supramolecular complex with an amide-type tripodal ligand]. Acta Chimica Sinica (in Chinese). 64 (5): 444–448.
- ^ a b Curzon A.E.; Chlebek H.G. (1973). "The observation of face centred cubic Gd, Tb, Dy, Ho, Er and Tm in the form of thin films and their oxidation". J. Phys. F. 3 (1): 1–5. Bibcode:1973JPhF....3....1C. doi:10.1088/0305-4608/3/1/009.
- ^ a b c Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- ^ Zhang, J.; Von Dreele, R.B.; Eyring, L. (May 1993). "The Structures of Tb7O12 and Tb11O20". Journal of Solid State Chemistry. 104 (1): 21–32. Bibcode:1993JSSCh.104...21Z. doi:10.1006/jssc.1993.1138.
- ^ a b c d Schäfer, W.; Will, G.; Müller-Vogt, G. (1979). "Refinement of the crystal structure of terbium arsenate TbAsO4 at 77 K and 5 K by profile analysis from neutron diffraction powder data". Acta Crystallographica Section B: Structural Crystallography and Crystal Chemistry. 35 (3): 588–592. doi:10.1107/S0567740879004210.
- ^ a b Siqueira, Kisla P. F.; Lima, Patrícia P.; Ferreira, Rute A. S.; Carlos, Luís D.; Bittar, Eduardo M.; Granado, Eduardo; González, Juan Carlos; Abelenda, Arturo; Moreira, Roberto L.; Dias, Anderson (2014). "Lanthanide Orthoantimonate Light Emitters: Structural, Vibrational, and Optical Properties". Chemistry of Materials. 26 (22): 6351–6360. doi:10.1021/cm502504b.
- ^ Olcese, Giorgio L. Structure and magnetic properties of MX compounds from terbium and metalloids of the Groups V and VI. Atti Accad. Nazi. Lincei Rend. Classe Sci. Fis. Mat. e Nat., 1961. 30: 195-200.
- ^ Pribyl'skaya, N. Yu; Orlova, I. G.; Shkabura, O. N.; Eliseev, A. A. (March 1985). "Синтез и исследование физико-химических свойств селенидов тербия" [Synthesis and study of physicochemical properties of terbium selenides]. Zhurnal Neorganicheskoj Khimii (in Russian). 30 (3): 603–606. OSTI 5087056. INIST 9092162.
- ^ a b Chen Shouchun. Important Inorganic Chemical Reactions. Shanghai Science and Technology Press, 1994. pp 1304-1305.
- ^ Hartmut Bergmann, Leopold Gmelin (1986). Gmelin Handbook of Inorganic Chemistry, System Number 39. Springer-Verlag. p. 397. ISBN 978-3-540-93525-4.
- ^ Teixeira, J.A.; Nunes, W.D.G.; do Nascimento, A.L.C.S.; Colman, T.A.D.; Caires, F.J.; Gálico, D.A.; Ionashiro, M. (2016). "Synthesis, thermoanalytical, spectroscopic study and pyrolysis of solid rare earth complexes (Eu, Gd, Tb and Dy) with p -aminobenzoic acid". Journal of Analytical and Applied Pyrolysis. 121: 267–274. doi:10.1016/j.jaap.2016.08.006. hdl:11449/178331.
- ^ Edelmann, F.T.; Poremba, P. (1967). Herrmann, W.A. (ed.). Synthetic Methods of Organometallic and Inorganic Chemistry. Vol. 6. Stuttgart: Georg Thieme Verlag. ISBN 3-13-103071-2.
- ^ Lei, M.; Zhao, H.Z.; Yang, H.; Song, B.; Cao, L.Z.; Li, P.G.; Tang, W.H. (2008). "Syntheses of metal nitrides, metal carbides and rare-earth metal dioxymonocarbodiimides from metal oxides and dicyandiamide". Journal of Alloys and Compounds. 460 (1–2): 130–137. doi:10.1016/j.jallcom.2007.05.076.
- ^ G. J. McCarthy (October 1971). "Crystal data on C-type terbium sesquioxide (Tb
2O
3)". Journal of Applied Crystallography. 4 (5): 399–400. doi:10.1107/S0021889871007295. - ^ Reidar Haugsrud; Yngve Larring & Truls Norby (December 2005). "Proton conductivity of Ca-doped Tb
2O
3". Solid State Ionics. 176 (39–40). Elsevier B.V.: 2957–2961. doi:10.1016/j.ssi.2005.09.030. - ^ Inorganic Chemistry Series. pp 244-257
- ^ 杨, 汝栋; 刘, 建民; 马, 太儒 (1983). "用化学氧化法从水溶液中制备Tb(IV)水合氧化物及其性质的研究 - 中国知网". 兰州大学学报 (1): 71–80. doi:10.13885/j.issn.0455-2059.1983.01.010.
- ^ Brauer, G.; Pfeiffer, B. (November 1966). "Über Mischkristalle zwischen Praseodymdioxid und Terbiumdioxid". Journal für Praktische Chemie. 34 (1–4): 23–29. doi:10.1002/prac.19660340103.
- ^ Orlova, I. G.; Eliseev, A. A. (1983). "Физико-химическое исследование взаимоде ствия серы с тэрбием" [Physicochemical investigation into reaction of sulfur with terbium]. Zhurnal Neorganicheskoj Khimii (in Russian). 28 (1): 65–68. OSTI 5032526. INIST PASCAL83X0237088.
- ^ a b Andrrev, O.V.; Razumkova, I.A.; Boiko, A.N. (2018). "Synthesis and thermal stability of rare earth compounds REF3 , REF3 · n H2O and (H3O)RE3F10 · n H2O (RE = Tb − Lu, Y), obtained from sulphide precursors". Journal of Fluorine Chemistry. 207: 77–83. doi:10.1016/j.jfluchem.2017.12.001.
- ^ Grundmeier, Thorsten; Urland, Werner (November 1997). "Zur Kristallstruktur von Tb2Se3". Zeitschrift für anorganische und allgemeine Chemie. 623 (11): 1744–1746. doi:10.1002/zaac.19976231113.
- ^ a b Terbium: reactions of elements Archived 2022-09-20 at the Wayback Machine. WebElements. [2017-4-10]
- ^ Inorganic Chemistry Series. pp 210-215. 2. Halides
- ^ Cotton (2007). Advanced inorganic chemistry (6th ed.). Wiley-India. p. 1128. ISBN 978-81-265-1338-3.
- ^ Rau, J. V.; Chilingarov, N. S.; Leskiv, M. S.; Sukhoverkhov, V. F.; Rossi Albertini, V.; Sidorov, L. N. (August 2001). "Transition and rare earth metal fluorides as thermal sources of atomic and molecular fluorine". Le Journal de Physique IV. 11 (PR3): Pr3–109–Pr3-113. doi:10.1051/jp4:2001314.
- ^ G. Meyer; Lester R. Morss (1991). Synthesis of Lanthanide and Actinide Compounds. Springer Science & Business Media. p. 60. ISBN 978-0-7923-1018-1.
- ^ Gaumet, V.; Avignant, D. (1997). "Caesium Pentafluoroterbate, CsTbF5". Acta Crystallographica Section C: Crystal Structure Communications. 53 (9): 1176–1178. doi:10.1107/S0108270197005556.
- ^ Largeau, E.; El-Ghozzi, M.; Métin, J.; Avignant, D. (1997). "β-BaTbF6". Acta Crystallographica Section C: Crystal Structure Communications. 53 (5): 530–532. doi:10.1107/S0108270196014527.
- ^ 无机化学丛书. pp 338. 2.3.7 稀土元素有机化合物.
- ^ a b Hagen, A. P. (2009). Inorganic Reactions and Methods, The Formation of Bonds to Elements of Group IVB (C, Si, Ge, Sn, Pb) (Part 4). John Wiley & Sons. ISBN 978-0-470-14547-0.[page needed]
- ^ Wei, D.Y.; Zheng, Y.-Q. (2003). "Crystal structure of terbium sulfate octahydrate, Tb2(SO4)3 · 8H2O, and of dysprosium sulfate octahydrate, Dy2(SO4)3 · 8H2O". Zeitschrift für Kristallographie - New Crystal Structures. 218 (JG): 23–24. doi:10.1524/ncrs.2003.218.jg.23. S2CID 98631742.
- ^ 无机化合物合成手册. pp 258-259. 819 硫酸盐.
- ^ "稀土硝酸盐的制法、性质及结构".
{{cite journal}}: Cite journal requires|journal=(help) - ^ 无机化合物合成手册. pp 260-261. 820 硝酸盐.
- ^ Di, Weihua; Wang, Xiaojun; Zhao, Haifeng (2007). "Synthesis and Characterization of LnPO4 · nH2O (Ln = La, Ce, Gd, Tb, Dy) Nanorods and Nanowires". Journal of Nanoscience and Nanotechnology. 7 (10): 3624–3628. doi:10.1166/jnn.2007.847. PMID 18330183.
- ^ Kalieva, K.; Stretkova, Z. V.; Babynina, N. A.; Gordienko, L. N.; Votoyarov, M.; Yakimov; Sakavov, I. E.; Mustaev, A. K. X-ray diffraction studies of praseodymium, neodymium, europium, terbium, dysprosium, erbium, holmium, and terbium orthophosphates. (in Russian) Tr. Kirg. Univ., Ser. Khim. Nauk, 1972. (2): 84-89.
- ^ Gabisoniya, Ts. D.; Nanobashvili, E. M. (1980). "Synthesis of rare earth metal arsenates". Soobshcheniya Akademii Nauk Gruzinskoi SSR. 97 (2): 345–348. ISSN 0002-3167.[verification needed]
- ^ Sastry, R.L.N.; Yoganarasimhan, S.R.; Mehrotra, P.N.; Rao, C.N.R. (1966). "Preparation, characterization and thermal decomposition of praseodymium, terbium and neodymium carbonates". Journal of Inorganic and Nuclear Chemistry. 28 (5): 1165–1177. doi:10.1016/0022-1902(66)80442-6.
- ^ Fulle, Kyle; Sanjeewa, Liurukara D.; McMillen, Colin D.; Wen, Yimei; Rajamanthrilage, Apeksha C.; Anker, Jeffrey N.; Chumanov, George; Kolis, Joseph W. (2017). "One-Pot Hydrothermal Synthesis of TbIII13(GeO4)6O7(OH) and K2TbIVGe2O7: Preparation of a Stable Terbium(4+) Complex". Inorganic Chemistry. 56 (11): 6044–6047. doi:10.1021/acs.inorgchem.7b00821. OSTI 1596455. PMID 28537716.
- ^ Manabe, Kazuo; Ogawa, Makoto (1982). "Thermal Decomposition of Terbium(III) Acetate Tetrahydrate". Nippon Kagaku Kaishi. 1982 (4): 694–696. doi:10.1246/nikkashi.1982.694.
- ^ Zeng, Yubin; Liang, Yingfang (2013). "A General Approach to Spindle-Assembled Lanthanide Borate Nanocrystals and Their Photoluminescence upon Eu3+/Tb3+ Doping". Inorganic Chemistry. 52 (16): 9590–9596. doi:10.1021/ic401299h. PMID 23899367.
- ^ Cao, Li Li; Chen, Yi Yong; Lin, Chan Juan; Shen, Ze Bin; Guo, Fei Yun; Ye, Jing; Chen, Jian Zhong (2011). "Preparation of TbBO3 Powder and Growth of TbBO3 Crystal". Advanced Materials Research. 306–307: 416–422. doi:10.4028/www.scientific.net/AMR.306-307.416. S2CID 138908676.
- ^ Ritter, C; Balaev, A; Vorotynov, A; Petrakovskii, G; Velikanov, D; Temerov, V; Gudim, I (2007). "Magnetic structure, magnetic interactions and metamagnetism in terbium iron borate TbFe3(BO3)4: a neutron diffraction and magnetization study". Journal of Physics: Condensed Matter. 19 (19): 196227. Bibcode:2007JPCM...19s6227R. doi:10.1088/0953-8984/19/19/196227. S2CID 93818255.
- ^ Kadomtseva, A. M.; Popov, Yu. F.; Vorob'ev, G. P.; Kostyuchenko, N. V.; Popov, A. I.; Mukhin, A. A.; Ivanov, V. Yu.; Bezmaternykh, L. N.; Gudim, I. A.; Temerov, V. L.; Pyatakov, A. P.; Zvezdin, A. K. (2014). "High-temperature magnetoelectricity of terbium aluminum borate: The role of excited states of the rare-earth ion". Physical Review B. 89 (1): 014418. Bibcode:2014PhRvB..89a4418K. doi:10.1103/PhysRevB.89.014418.
- ^ Schäfer, Marion C.; Nikelski, Tanja; Schleid, Thomas (2013). "Syntheses and crystal structures of the novel oxide chloride oxoborates Ln4O4Cl[BO3] (Ln = Eu–Tm)". Zeitschrift für Kristallographie - Crystalline Materials. 228 (9). doi:10.1524/zkri.2013.1648. S2CID 100606856.
- ^ a b Lin, Hui; Zhou, Shengming; Teng, Hao (2011). "Synthesis of Tb3Al5O12 (TAG) transparent ceramics for potential magneto-optical applications". Optical Materials. 33 (11): 1833–1836. Bibcode:2011OptMa..33.1833L. doi:10.1016/j.optmat.2011.06.017.
- ^ a b Jin, Weizhao; Ding, Jingxin; Guo, Li; Gu, Qi; Li, Chun; Su, Liangbi; Wu, Anhua; Zeng, Fanming (2018). "Growth and performance research of Tb 3 Ga 5 O 12 magneto-optical crystal". Journal of Crystal Growth. 484: 17–20. Bibcode:2018JCrGr.484...17J. doi:10.1016/j.jcrysgro.2017.12.024.
- ^ a b Löw, Ute; Zherlitsyn, Sergei; Araki, Koji; Akatsu, Mitsuhiro; Nemoto, Yuichi; Goto, Terutaka; Zeitler, Uli; Lüthi, Bruno (2014). "Magneto-Elastic Effects in Tb3Ga5O12". Journal of the Physical Society of Japan. 83 (4): 044603. Bibcode:2014JPSJ...83d4603L. doi:10.7566/JPSJ.83.044603.
- ^ "Terbium Phosphide TbP". materialsproject.org. Retrieved 24 December 2021.
- ^ Dubey, Ritu; Singh, Sadhna; Kaur, Gurusharan (2021-01-01). "Structural analysis of terbium monopnictides under high pressure". Solid State Communications. 338: 114465. Bibcode:2021SSCom.33814465D. doi:10.1016/j.ssc.2021.114465.
- ^ Hafemeister, D. W.; Flygare, W. H. (August 1965). "Outer-Shell Overlap Integrals as a Function of Distance for Halogen—Halogen, Halogen—Alkali, and Alkali—Alkali Ions in the Alkali Halide Lattices". The Journal of Chemical Physics. 43 (3): 795–800. Bibcode:1965JChPh..43..795H. doi:10.1063/1.1696846.
- ^ Rowley, Adrian T.; Parkin, Ivan P. (1 January 1993). "Convenient synthesis of lanthanide and mixed lanthanide phosphides by solid-state routes involving sodium phosphide". Journal of Materials Chemistry. 3 (7): 689–692. doi:10.1039/JM9930300689.
- ^ Adachi, Takafumi; Shirotani, Ichimin; Hayashi, Junichi; Shimomura, Osamu (28 December 1998). "Phase transitions of lanthanide monophosphides with NaCl-type structure at high pressures". Physics Letters A. 250 (4–6): 389–393. Bibcode:1998PhLA..250..389A. doi:10.1016/S0375-9601(98)00840-8.
- ^ Raffius, G.; Kötzler, J. (February 1983). "Field-dependence of the first-order phase transition in terbium phosphide". Physics Letters A. 93 (8): 423–425. Bibcode:1983PhLA...93..423R. doi:10.1016/0375-9601(83)90477-2.
- ^ Wheeler, Scott L. (2002-10-29). "PRC Espionage leads to "Terf" war: investigators say China placed students in American universities to gain secret information about an exotic material with valuable industrial and military uses". Insight on the News Newspaper. Archived from the original on 2012-07-16. Retrieved 2010-04-08.
- ^ Sandlund, L.; Fahlander, M.; Cedell, T.; Clark, A. E.; Restorff, J. B.; Wun-Fogle, M. (15 May 1994). "Magnetostriction, elastic moduli, and coupling factors of composite Terfenol-D". Journal of Applied Physics. 75 (10): 5656–5658. Bibcode:1994JAP....75.5656S. doi:10.1063/1.355627.
- ^ Lim, S.H.; Kim, S.R.; Kang, S.Y.; Park, J.K.; Nam, J.T.; Son, Derac (January 1999). "Magnetostrictive properties of polymer-bonded Terfenol-D composites". Journal of Magnetism and Magnetic Materials. 191 (1–2): 113–121. Bibcode:1999JMMM..191..113L. doi:10.1016/S0304-8853(98)00315-1.
- ^ Verhoeven, J. D.; Ostenson, J. E.; Gibson, E. D.; McMasters, O. D. (1989-07-15). "The effect of composition and magnetic heat treatment on the magnetostriction of TbxDy1−xFey twinned single crystals". Journal of Applied Physics. 66 (2): 772–779. Bibcode:1989JAP....66..772V. doi:10.1063/1.343496.
- ^ Elder, Eleanor S (1980). "Sir William Crookes, Victorium, and the Library of Congress". Journal of Chemical Education. 57 (6): 421. Bibcode:1980JChEd..57..421E. doi:10.1021/ed057p421.
- ^ Fontani, Marco; Costa, Mariagrazia; Orna, Mary Virginia (2014). The Lost Elements: The Periodic Table's Shadow Side. Oxford University Press. pp. 202–203. ISBN 978-0-19-938336-8.
- ^ Brock, William Hodson (2008). William Crookes (1832-1919) and the Commercialization of Science. Ashgate Publishing. pp. 321–325. ISBN 978-0-7546-6322-5.
- ^ Caro, Paul (1998-06-01). "Rare earths in luminescence". Rare earths. Editorial Complutense. pp. 323–325. ISBN 978-84-89784-33-8. Archived from the original on 2020-03-13. Retrieved 2019-07-06.
- ^ Manwani, Krishna; Chelvane, Arout J.; Panda, Emila (2018). "Oxidation of TbFe2: Microstructure of oxide-film by both theory and experiment". Corrosion Science. 130: 153–160. doi:10.1016/j.corsci.2017.10.030.
- ^ Sigma-Aldrich Co., product no. {{{id}}}.
- ^ "加替沙星铽敏化化学发光与应用".
{{cite journal}}: Cite journal requires|journal=(help) - ^ "Terbium Phosphide". American Elements. Retrieved 9 January 2022.
- ^ Di, Weihua; Li, Jie; Shirahata, Naoto; Sakka, Yoshio; Willinger, Marc-Georg; Pinna, Nicola (2011). "Photoluminescence, cytotoxicity and in vitro imaging of hexagonal terbium phosphatenanoparticles doped with europium". Nanoscale. 3 (3): 1263–1269. Bibcode:2011Nanos...3.1263D. doi:10.1039/C0NR00673D. PMID 21135975.
- ^ "Fuel Injector Patent". Retrieved 2011-02-18.
- ^ a b c "Active Signal Technologies Terfenol-D Transducer and Actuator Designs". www.activesignaltech.com. Retrieved 2018-12-09.
- ^ Houqing, Zhu; Jianguo, Liu; Xiurong, Wang; Yanhong, Xing; Hongping, Zhang (1997-08-01). "Applications of Terfenol-D in China". Journal of Alloys and Compounds. 258 (1–2): 49–52. doi:10.1016/S0925-8388(97)00068-6.
- ^ "Fuel Injector Patent". Retrieved 2011-02-18.