Transcranial direct-current stimulation
| Transcranial direct-current stimulation | |
|---|---|
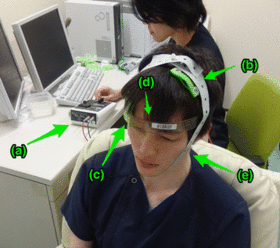 Anodal tDCS administration. Anodal (b) and cathodal (c) electrodes with 35 cm2 size are put on F3 and right supraorbital region, respectively. A head strap is used (d) for convenience and reproducibility, and a rubber band (e) for reducing resistance. | |
| MeSH | D065908 |
Transcranial direct current stimulation (tDCS) is a form of neuromodulation that uses constant, low direct current delivered via electrodes on the head. It was originally developed to help patients with brain injuries or neuropsychiatric conditions such as major depressive disorder. It can be contrasted with cranial electrotherapy stimulation, which generally uses alternating current the same way, as well as transcranial magnetic stimulation.[1]
Research shows increasing evidence for tDCS as a treatment for depression.[2][3][4] There is mixed evidence about whether tDCS is useful for cognitive enhancement in healthy people. There is no strong evidence that tDCS is useful for memory deficits in Parkinson's disease and Alzheimer's disease,[5] non-neuropathic pain,[6] nor for improving arm or leg functioning and muscle strength in people recovering from a stroke.[7] There is emerging supportive evidence for tDCS in the management of schizophrenia – especially for negative symptoms.[8][9]
Efficacy
[edit]Depression
[edit]There is some evidence tDCS might be of moderate benefit as treatment for mild and moderate Depression,[10] major depressive disorder[11] and treatment-resistant depression.[12]
Other medical use
[edit]Recent research on tDCS has shown promising results in treating other mental health conditions such as anxiety[10] and PTSD.[13] More research is required on the topic. There is also evidence that tDCS is useful in treating neuropathic pain after spinal cord injury.[14] There is evidence of very low to moderate quality that tDCS can improve activities of daily living assessment in the short-term after stroke.[15][7]
Transcranial direct-current stimulaiton is also used to augment speech therapy in patients with acquired language disorders like aphasia, or to help maintain language abilities in the case of primary progressive aphasia, a neurodegenerative condition.[16]
Safety
[edit]According to the British National Institute for Health and Care Excellence (NICE), the evidence on tDCS for depression raises no major safety concerns.[17]
As of 2017, at stimulation up to 60 min and up to 4 mA over two weeks, adverse effects include skin irritation, a phosphene at the start of stimulation, nausea, headache, dizziness, and itching under the electrode. Typical treatment sessions lasting for about 20–30 minutes repeated daily for several weeks in the treatment of depression.[18] Adverse effects of long term treatment were not known as of 2017.[19] Nausea most commonly occurs when the electrodes are placed above the mastoid for stimulation of the vestibular system. A phosphene is a brief flash of light that can occur if an electrode is placed near the eye.[20][21]
People susceptible to seizures, such as people with epilepsy should not receive tDCS.[20] Studies have been completed to determine the current density at which overt brain damage occurs in rats. It was found that in cathodal stimulation, a current density of 142.9 A/m2 delivering a charge density of 52400 C/m2 or higher caused a brain lesion in the rat. This is over two orders of magnitude higher than protocols that were in use as of 2009.[22][23][24]
Mechanism of action
[edit]tDCS stimulates and activates brain cells by delivering electrical signals. The lasting modulation of cortical excitability produced by tDCS makes it an effective solution to facilitate rehabilitation and treat a range of neuropsychiatric disorders.[25] The way that the stimulation changes brain function is either by causing the neuron’s resting membrane potential to depolarize or hyperpolarize. When positive stimulation (anodal tDCS) is delivered, the current causes a depolarization of the resting membrane potential, which increases neuronal excitability and allows for more spontaneous cell firing. When negative stimulation (cathodal tDCS) is delivered, the current causes a hyperpolarization of the resting membrane potential. This decreases neuron excitability due to the decreased spontaneous cell firing.[20][26]
In case of treating depression, tDCS currents specifically target the left side of dorsolateral prefrontal cortex (DLPFC) located in the frontal lobe. Left DLPFC has been shown to be associated with lower activity in the depressed population.[27][11]
tDCS is able to achieve cortical changes even after the stimulation is ended. The duration of this change depends on the length of stimulation as well as the intensity of stimulation. The effects of stimulation increase as the duration of stimulation increases or the strength of the current increases.[28] tDCS has been proposed to promote both long term potentiation and long term depression,[20][26] and further research is needed for validation.
Operation
[edit]Transcranial direct current stimulation works by sending constant, low direct current through the electrodes. When these electrodes are placed in the region of interest, the current induces intracerebral current flow. This current flow then either increases or decreases the neuronal excitability in the specific area being stimulated based on which type of stimulation is being used. This change of neuronal excitability leads to alteration of brain function, which can be used in various therapies as well as to provide more information about the functioning of the human brain.[20]
Parts
[edit]Transcranial direct current stimulation is a relatively simple technique requiring only a few parts. These include two electrodes and a battery-powered device that delivers constant current. Control software can also be used in experiments that require multiple sessions with differing stimulation types so that neither the person receiving the stimulation nor the experimenter knows which type is being administered. Each device has an anodal, positively charged electrode and a cathodal, negative electrode. Current is "conventionally" described as flowing from the positive anode, through the intervening conducting tissue, to the cathode, creating a circuit. Note that in traditional electric circuits constructed from metal wires, charge drift is created by the motion of negatively charged electrons, which actually flow from cathode to anode. However, in biological systems, such as the head, current is usually created by the flow of ions, which may be positively or negatively charged – positive ions will flow towards the cathode; negative ions will flow toward the anode. The device may control the current as well as the duration of stimulation.[29]
Setup
[edit]To set up the tDCS device, the electrodes and the skin need to be prepared. This ensures a low resistance connection between the skin and the electrode. The careful placement of the electrodes is crucial to successful tDCS technique. The electrode pads come in various sizes with benefits to each size. A smaller sized electrode achieves a more focused stimulation of a site while a larger electrode ensures that the entirety of the region of interest is being stimulated.[30] If the electrode is placed incorrectly, a different site or more sites than intended may be stimulated resulting in faulty results.[20] One of the electrodes is placed over the region of interest and the other electrode, the reference electrode, is placed in another location in order to complete the circuit. This reference electrode is usually placed on the neck or shoulder of the opposite side of the body than the region of interest. Since the region of interest may be small, it is often useful to locate this region before placing the electrode by using a brain imaging technique such as fMRI or PET.[20] Once the electrodes are placed correctly, the stimulation can be started. Many devices have a built-in capability that allows the current to be "ramped up" or increased gradually until the necessary current is reached. This decreases the amount of stimulation effects felt by the person receiving the tDCS.[31] After the stimulation has been started, the current will continue for the amount of time set on the device and then will automatically be shut off. Recently a new approach has been introduced where instead of using two large pads, multiple (more than two) smaller sized gel electrodes are used to target specific cortical structures. This new approach is called High Definition tDCS (HD-tDCS).[30][32] In a pilot study, HD-tDCS was found to have greater and longer lasting motor cortex excitability changes than sponge tDCS.[33]
Types of stimulation
[edit]There are three different types of stimulation: anodal, cathodal, and sham. The anodal stimulation is positive (V+) stimulation that increases the neuronal excitability of the area being stimulated. Cathodal (V−) stimulation decreases the neuronal excitability of the area being stimulated. Cathodal stimulation can treat psychiatric disorders that are caused by the hyper-activity of an area of the brain.[34] Sham stimulation is used as a control in experiments. Sham stimulation emits a brief current but then remains off for the remainder of the stimulation time. With sham stimulation, the person receiving the tDCS does not know that they are not receiving prolonged stimulation. By comparing the results in subjects exposed to sham stimulation with the results of subjects exposed to anodal or cathodal stimulation, researchers can see how much of an effect is caused by the current stimulation, rather than by the placebo effect.
At-home administration
[edit]Recently, tDCS devices are being researched and created intended for at-home use – ranging from treating medical conditions such as depression to enhancing general cognitive well-being.[35][36] Clinical trials are needed to establish the efficacy, feasibility and acceptability of home-based tDCS treatment.[37]
History
[edit]The basic design of tDCS, using direct current (DC) to stimulate the area of interest, has existed for over 100 years. There were a number of rudimentary experiments completed before the 19th century using this technique that tested animal and human electricity. Luigi Galvani and Alessandro Volta were two such researchers that utilized the technology of tDCS in their explorations of the source of animal cell electricity [citation needed]. It was due to these initial studies that tDCS was first brought into the clinical scene. In 1801, Giovanni Aldini (Galvani's nephew) started a study in which he successfully used the technique of direct current stimulation to improve the mood of melancholy patients.[38]
There was a brief rise of interest in transcranial direct current stimulation in the 1960s when studies by researcher D. J. Albert proved that the stimulation could affect brain function by changing the cortical excitability.[39] He also discovered that positive and negative stimulation had different effects on the cortical excitability.[40]
Research continued, further fueled by knowledge gained from other techniques like TMS and fMRI.[28][20]
Comparison to other devices
[edit]
In transcranial magnetic stimulation (TMS), an electric coil is held above the region of interest on the scalp that uses rapidly changing magnetic fields to induce small electrical currents in the brain. There are two types of TMS: repetitive TMS and single pulse TMS. Both are used in research therapy but effects lasting longer than the stimulation period are only observed in repetitive TMS. Similar to tDCS, an increase or decrease in neuronal activity can be achieved using this technique, but the method of how this is induced is very different. Transcranial direct current stimulation has the two different directions of current that cause the different effects. Increased neuronal activity is induced in repetitive TMS by using a higher frequency and decreased neuronal activity is induced by using a lower frequency.[29]
Variants related to tDCS include tACS, tPCS and transcranial random noise stimulation (tRNS), a group of technologies commonly referred to as transcranial electrical stimulation, or TES.[41]
Research
[edit]Depression
[edit]Determining the safety and effectiveness of tDCS treatment for people with depression is being investigated:
- A systematic review of placebo-controlled trials investigating tDCS treatment for major depressive disorder was published 2020.[11] The meta-analysis collated results across nine eligible studies (572 participants) up until December 2018 to estimate odds ratio (OR) and number needed to treat (NNT) of response and remission, and depression improvement. The results showed statistically superior efficacy of active tDCS compared to sham for nine eligible studies (572 participants), presenting moderate/high certainty of evidence, were included. Active tDCS was significantly superior to sham for response (30.9% vs. 18.9% respectively; OR = 1.96, 95%CI [1.30–2.95], NNT = 9), remission (19.9% vs. 11.7%, OR = 1.94 [1.19–3.16], NNT = 13) and depression improvement (effect size of β = 0.31, [0.15–0.47]).[11]
- A 2016 meta-analysis showed that 34% of people treated with tDCS showed at least 50% symptom reduction (compared to 19% placebo).[42]
- A 2017 study conducted by Brunoni showed 6-weeks of tDCS treatment resulted in reduction of at least half of depression symptoms in 41% of depressed people (vs. 22% placebo and 47% antidepressants).[43]
- In 2015, the British National Institute for Health and Care Excellence (NICE) found tDCS to be safe and to appear effective for depression treatment. Up until 2014, there have been several small randomized clinical trials (RCT) in major depressive disorder (MDD); most found alleviation of depressive symptoms. There have been only two RCTs in treatment-resistant MDD; both were small, and one found an effect and the other did not.[44] One meta-analysis of the data focused on reduction in symptoms and found an effect compared to sham treatment, but another that was focused on relapse found no effect compared to sham.[44]
Other disorders
[edit]Cognition
[edit]There is mixed evidence about whether tDCS is useful for cognitive enhancement in healthy people. Several reviews have found evidence of small yet significant cognitive improvements.[45][46][47][48] Other reviews found no evidence at all,[49][50] although one of them[50] has been criticized for overlooking within-subject effects[51] and evidence from multiple-session tDCS trials. However, the original authors addressed these raised concerns in a further analysis and continued to find no evidence of impact [52]
A 2015 review of results from hundreds of tDCS experiments found that there was no statistically conclusive evidence to support any net cognitive effect, positive or negative, of single session tDCS in healthy populations – there is no evidence that tDCS is useful for cognitive enhancement.[50] A second study by the same authors found there was little-to-no statistically reliable impact of tDCS on any neurophysiologic outcome.[49]
Parkinson's, Alzheimer's disease, and schizophrenia
[edit]There is no strong evidence that tDCS is useful for memory deficits in Alzheimer's disease,[5] schizophrenia,[53] non-neuropathic pain.[6] A few clinical trials have been conducted on the use of tDCS to ameliorate memory deficits in Parkinson's disease and Alzheimer's disease and healthy subjects, with mixed results.[5] Research conducted as of 2013 in schizophrenia, has found that while large effect sizes were initially found for symptom improvement, later and larger studies have found smaller effect sizes (see also section on use of tDCS in psychiatric disorders below).[53] Studies have mostly concentrated on positive symptoms like auditory hallucinations; research on negative symptoms is lacking.[53]
Stroke
[edit]There is no strong evidence that tDCS can help improve upper limb function after stroke.[54][55] In stroke, research conducted as of 2014, has found that tDCS is not effective for improving upper limb function after stroke.[54][55] While some reviews have suggested an effect of tDCS for improving post-stroke aphasia, a 2015 Cochrane review could find no improvement from combining tDCS with conventional treatment.[56][55][57] Research conducted as of 2013 suggests that tDCS may be effective for improve vision deficits following stroke.[55]
Motor Learning and Memory Function
There is evidence that certain tDCS montages can increase learning rates for particular tasks in healthy individuals, namely motor tasks and memory function.[58] However, reproducibility remains to be fully tested across studies and standardization for these kinds of studies has not been implemented fully, though an attempt at formalizing standards was released in 2017.[58]
Other
[edit]Research conducted as of 2012 on the use of tDCS to treat pain, found that the research has been of low quality and cannot be used as a basis to recommend use of tDCS to treat pain.[6] In chronic pain following spinal cord injury, research is of high quality and has found tDCS to be ineffective.[59] tDCS has also been studied in addiction.[60][61] There is some moderate (level B) evidence to indicate that, in addition to treating major depressive disorder, tDCS may also be appropriate to treat fibromyalgia, and craving disorders.[62]
tDCS has been used in neuroscience research, particularly to try to link specific brain regions to specific cognitive tasks[63] or psychological phenomena.[64] The cerebellum has been a focus of research, due to its high concentration of neurons, its location immediately below the skull, and its multiple reciprocal anatomical connections to motor and associative parts of the brain.[65] Most such studies focus on the impact of cerebellar tDCS on motor, cognitive, and affective functions in healthy and patient populations, but some also employ tDCS over the cerebellum to study the functional connectivity of the cerebellum to other areas of the brain.[66]
Limitations
[edit]While growing literature shows the efficacy of transcranial direct current stimulation (tDCS) for treating nervous diseases such as acute depressive episodes, the lack of knowledge about the nature of this treatment at the cell level[1] raises concerns regarding possible adverse effects that would appear long after the treatment has ended.
First, tDCS therapy involves exposure of the brain to an intense electric field, which is several times and even orders of magnitude higher than natural ones in the brain.[67][68] While the therapeutic effect is observed in a short period of months, the impact of the electric fields on the brain, specifically on the treated neuronal structures, is a question of further long-term research.
Second, tDCS brain tissue stimulation targets a large area of poorly characterized tissue.[67][69] Therefore, it is unclear whether electrical fields reach only the neural structures of the brain that need treatment. The radiation can destroy healthy cells which don't need treatment during tDCS therapy.[69]
Regulatory approvals
[edit]tDCS is a CE approved treatment for major depressive disorder (MDD) in the UK, EU, Australia, and Mexico. As of 2015, tDCS has not been approved for any use by the US FDA.[57] An FDA briefing document prepared in 2012 stated that "there is no regulation for therapeutic tDCS".[70]
See also
[edit]References
[edit]- ^ a b Rosa MA, Lisanby SH (January 2012). "Somatic treatments for mood disorders". Neuropsychopharmacology. 37 (1): 102–116. doi:10.1038/npp.2011.225. PMC 3238088. PMID 21976043.
- ^ Brunoni AR, Moffa AH, Fregni F, Palm U, Padberg F, Blumberger DM, et al. (June 2016). "Transcranial direct current stimulation for acute major depressive episodes: meta-analysis of individual patient data". The British Journal of Psychiatry. 208 (6): 522–531. doi:10.1192/bjp.bp.115.164715. PMC 4887722. PMID 27056623.
Our findings indicates two potential applications for tDCS in the therapeutic arsenal for depression: in primary care settings and as a non-pharmacological, neuromodulatory therapy for depression.
- ^ Mutz J, Vipulananthan V, Carter B, Hurlemann R, Fu CH, Young AH (March 2019). "Comparative efficacy and acceptability of non-surgical brain stimulation for the acute treatment of major depressive episodes in adults: systematic review and network meta-analysis". BMJ. 364: l1079. doi:10.1136/bmj.l1079. PMC 6435996. PMID 30917990.
we found tDCS to be efficacious across outcomes in both pairwise and network meta-analyses.
- ^ "Transcranial direct current stimulation (tDCS) for depression". The National Institute for Health and Care Excellence (NICE). UK. August 2015. Retrieved 10 November 2015.
- ^ a b c Bennabi D, Pedron S, Haffen E, Monnin J, Peterschmitt Y, Van Waes V (Sep 2014). "Transcranial direct current stimulation for memory enhancement: from clinical research to animal models". Frontiers in Systems Neuroscience. 8: 159. doi:10.3389/fnsys.2014.00159. PMC 4154388. PMID 25237299.
- ^ a b c Luedtke K, Rushton A, Wright C, Geiss B, Juergens TP, May A (June 2012). "Transcranial direct current stimulation for the reduction of clinical and experimentally induced pain: a systematic review and meta-analysis". The Clinical Journal of Pain. 28 (5): 452–461. doi:10.1097/AJP.0b013e31823853e3. PMID 22569218. S2CID 24612998.
- ^ a b Elsner B, Kugler J, Pohl M, Mehrholz J (November 2020). "Transcranial direct current stimulation (tDCS) for improving activities of daily living, and physical and cognitive functioning, in people after stroke". The Cochrane Database of Systematic Reviews. 2020 (11): CD009645. doi:10.1002/14651858.CD009645.pub4. PMC 8095012. PMID 33175411.
- ^ Liu Y, Gu N, Cao X, Zhu Y, Wang J, Smith RC, Li C (February 2021). "Effects of transcranial electrical stimulation on working memory in patients with schizophrenia: A systematic review and meta-analysis". Psychiatry Research. 296: 113656. doi:10.1016/j.psychres.2020.113656. PMID 33360429. S2CID 229367754.
- ^ Valiengo LD, Goerigk S, Gordon PC, Padberg F, Serpa MH, Koebe S, et al. (February 2020). "Efficacy and Safety of Transcranial Direct Current Stimulation for Treating Negative Symptoms in Schizophrenia: A Randomized Clinical Trial". JAMA Psychiatry. 77 (2): 121–129. doi:10.1001/jamapsychiatry.2019.3199. PMC 6802484. PMID 31617873.
- ^ a b Cheng, Ying-Chih; Kuo, Po-Hsiu; Su, Min-I; Huang, Wei-Lieh (2022-04-01). "The efficacy of non-invasive, non-convulsive electrical neuromodulation on depression, anxiety and sleep disturbance: a systematic review and meta-analysis". Psychological Medicine. 52 (5): 801–812. doi:10.1017/S0033291721005560. ISSN 0033-2917.
- ^ a b c d Moffa AH, Martin D, Alonzo A, Bennabi D, Blumberger DM, Benseñor IM, et al. (April 2020). "Efficacy and acceptability of transcranial direct current stimulation (tDCS) for major depressive disorder: An individual patient data meta-analysis". Progress in Neuro-Psychopharmacology & Biological Psychiatry. 99: 109836. doi:10.1016/j.pnpbp.2019.109836. hdl:1959.4/unsworks_81424. PMID 31837388. S2CID 209373871.
- ^ Li, John; Kung, Simon; Croarkin, Paul; Lapid, Maria (2024-04-01). "Transcranial Direct Current Stimulation (tDCS) for Treatment Resistant Depression (TRD): A Systematic Review". The American Journal of Geriatric Psychiatry. 32 (4): S99. doi:10.1016/j.jagp.2024.01.181.
- ^ Ahmadizadeh MJ, Rezaei M, Fitzgerald PB (November 2019). "Transcranial direct current stimulation (tDCS) for post-traumatic stress disorder (PTSD): A randomized, double-blinded, controlled trial". Brain Research Bulletin. 153: 273–278. doi:10.1016/j.brainresbull.2019.09.011. PMID 31560945. S2CID 202733889.
- ^ Soler MD, Kumru H, Pelayo R, Vidal J, Tormos JM, Fregni F, et al. (September 2010). "Effectiveness of transcranial direct current stimulation and visual illusion on neuropathic pain in spinal cord injury". Brain. 133 (9): 2565–2577. doi:10.1093/brain/awq184. PMC 2929331. PMID 20685806.
- ^ Elsner B, Kwakkel G, Kugler J, Mehrholz J (September 2017). "Transcranial direct current stimulation (tDCS) for improving capacity in activities and arm function after stroke: a network meta-analysis of randomised controlled trials". Journal of Neuroengineering and Rehabilitation. 14 (1): 95. doi:10.1186/s12984-017-0301-7. PMC 5598049. PMID 28903772.
- ^ Galletta, Elizabeth E.; Conner, Peggy; Vogel-Eyny, Amy; Marangolo, Paola (2016). "Use of TDCS in Aphasia Rehabilitation: A Systematic Review of the Behavioral Interventions Implemented with Noninvasive Brain Stimulation for Language Recovery". American Journal of Speech-Language Pathology. 25 (4S): S854 – S867. doi:10.1044/2016_AJSLP-15-0133. PMID 27997958.
- ^ "1 Recommendations | Transcranial direct current stimulation (tDCS) for depression | Guidance". The National Institute for Health and Care Excellence (NICE). UK. 26 August 2015. Retrieved 2020-05-12.
- ^ "3 The procedure | Transcranial direct current stimulation (tDCS) for depression | Guidance | NICE". The National Institute for Health and Care Excellence (NICE). UK. 26 August 2015. Retrieved 2020-05-12.
- ^ Poreisz C, Boros K, Antal A, Paulus W (May 2007). "Safety aspects of transcranial direct current stimulation concerning healthy subjects and patients". Brain Research Bulletin. 72 (4–6): 208–214. doi:10.1016/j.brainresbull.2007.01.004. PMID 17452283. S2CID 40861513.
- ^ a b c d e f g h Nitsche MA, Cohen LG, Wassermann EM, Priori A, Lang N, Antal A, et al. (July 2008). "Transcranial direct current stimulation: State of the art 2008". Brain Stimulation. 1 (3): 206–223. doi:10.1016/j.brs.2008.06.004. PMID 20633386. S2CID 16352598.
- ^ Antal A, Alekseichuk I, Bikson M, Brockmöller J, Brunoni AR, Chen R, et al. (September 2017). "Low intensity transcranial electric stimulation: Safety, ethical, legal regulatory and application guidelines". Clinical Neurophysiology. 128 (9): 1774–1809. doi:10.1016/j.clinph.2017.06.001. PMC 5985830. PMID 28709880.
- ^ Nitsche MA, Liebetanz D, Lang N, Antal A, Tergau F, Paulus W (November 2003). "Safety criteria for transcranial direct current stimulation (tDCS) in humans". Clinical Neurophysiology. 114 (11): 2220–2222, author reply 2222–2223. doi:10.1016/S1388-2457(03)00235-9. PMID 14580622. S2CID 46505774.
- ^ Liebetanz D, Koch R, Mayenfels S, König F, Paulus W, Nitsche MA (June 2009). "Safety limits of cathodal transcranial direct current stimulation in rats". Clinical Neurophysiology. 120 (6): 1161–1167. doi:10.1016/j.clinph.2009.01.022. PMID 19403329. S2CID 38005569.
- ^ Bikson M, Datta A, Elwassif M (June 2009). "Establishing safety limits for transcranial direct current stimulation". Clinical Neurophysiology. 120 (6): 1033–1034. doi:10.1016/j.clinph.2009.03.018. PMC 2754807. PMID 19394269.
- ^ Edwards D, Cortes M, Datta A, Minhas P, Wassermann EM, Bikson M (July 2013). "Physiological and modeling evidence for focal transcranial electrical brain stimulation in humans: a basis for high-definition tDCS". NeuroImage. 74: 266–75. doi:10.1016/j.neuroimage.2013.01.042. PMC 4359173. PMID 23370061.
- ^ a b Nitsche MA, Paulus W (September 2000). "Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation". The Journal of Physiology. 527 (Pt 3): 633–639. doi:10.1111/j.1469-7793.2000.t01-1-00633.x. PMC 2270099. PMID 10990547.
- ^ Fitzgerald PB (2011-10-24). "Transcranial direct current stimulation in the treatment of depression". Medicographia. Servier Laboratories. Archived from the original on 11 July 2020. Retrieved 2020-05-12.
- ^ a b Utz KS, Dimova V, Oppenländer K, Kerkhoff G (August 2010). "Electrified minds: transcranial direct current stimulation (tDCS) and galvanic vestibular stimulation (GVS) as methods of non-invasive brain stimulation in neuropsychology--a review of current data and future implications". Neuropsychologia. 48 (10): 2789–2810. doi:10.1016/j.neuropsychologia.2010.06.002. PMID 20542047. S2CID 14538856.
- ^ a b Sparing R, Mottaghy FM (April 2008). "Noninvasive brain stimulation with transcranial magnetic or direct current stimulation (TMS/tDCS)-From insights into human memory to therapy of its dysfunction". Methods. 44 (4): 329–337. doi:10.1016/j.ymeth.2007.02.001. PMID 18374276.
- ^ a b Datta A, Bansal V, Diaz J, Patel J, Reato D, Bikson M (October 2009). "Gyri-precise head model of transcranial direct current stimulation: improved spatial focality using a ring electrode versus conventional rectangular pad". Brain Stimulation. 2 (4): 201–7, 207.e1. doi:10.1016/j.brs.2009.03.005. PMC 2790295. PMID 20648973.
- ^ Viganò A, D'Elia TS, Sava SL, Auvé M, De Pasqua V, Colosimo A, et al. (March 2013). "Transcranial Direct Current Stimulation (tDCS) of the visual cortex: a proof-of-concept study based on interictal electrophysiological abnormalities in migraine". The Journal of Headache and Pain. 14 (1): 23. doi:10.1186/1129-2377-14-23. PMC 3620516. PMID 23566101.
- ^ Borckardt JJ, Bikson M, Frohman H, Reeves ST, Datta A, Bansal V, et al. (February 2012). "A pilot study of the tolerability and effects of high-definition transcranial direct current stimulation (HD-tDCS) on pain perception". The Journal of Pain. 13 (2): 112–120. doi:10.1016/j.jpain.2011.07.001. PMID 22104190.
- ^ Kuo HI, Bikson M, Datta A, Minhas P, Paulus W, Kuo MF, Nitsche MA (July 2013). "Comparing cortical plasticity induced by conventional and high-definition 4 × 1 ring tDCS: a neurophysiological study". Brain Stimulation. 6 (4): 644–648. doi:10.1016/j.brs.2012.09.010. PMID 23149292. S2CID 2283411.
- ^ Nitsche MA, Nitsche MS, Klein CC, Tergau F, Rothwell JC, Paulus W (April 2003). "Level of action of cathodal DC polarisation induced inhibition of the human motor cortex". Clinical Neurophysiology. 114 (4): 600–604. doi:10.1016/S1388-2457(02)00412-1. PMID 12686268. S2CID 20338096.
- ^ Riggs A, Patel V, Paneri B, Portenoy RK, Bikson M, Knotkova H (2018-05-22). "At-Home Transcranial Direct Current Stimulation (tDCS) With Telehealth Support for Symptom Control in Chronically-Ill Patients With Multiple Symptoms". Frontiers in Behavioral Neuroscience. 12: 93. doi:10.3389/fnbeh.2018.00093. PMC 5972211. PMID 29872381.
- ^ Thomson H. "Europe's first home brain-zap device for depression launched in UK". New Scientist. Retrieved 2020-05-12.
- ^ Woodham R, Rimmer RM, Mutz J, Fu CH (May 2021). "Is tDCS a potential first line treatment for major depression?". International Review of Psychiatry. 33 (3): 250–265. doi:10.1080/09540261.2021.1879030. PMID 33706656. S2CID 232209376.
- ^ Parent A (November 2004). "Aldini's Essay on Galvanism" (PDF). The Canadian Journal of Neurological Sciences. 31 (4): 576–584. doi:10.1017/S0317167100003851. PMID 15595271. S2CID 11048877. (Lanzarini pdf 5 of 9)
- Essai AJ (1804). Théorique et expérimental sur le galvanisme, avec une série d'expériences faites devant des commissaires de l'Institut national de France, et en divers amphithéâtres anatomiques de Londres [Theoretical and experimental on galvanism, with a series of experiments made before commissioners of the National Institute of France, and in various anatomical amphitheatres in London.] (in French). Paris: Fournier Fils.
- ^ Albert DJ (February 1966). "The effect of spreading depression on the consolidation of learning". Neuropsychologia. 4 (1): 49–64. doi:10.1016/0028-3932(66)90020-0. hdl:2027.42/33480.
- ^ Albert DJ (February 1966). "The effects of polarizing currents on the consolidation of learning". Neuropsychologia. 4 (1): 65–77. doi:10.1016/0028-3932(66)90021-2. hdl:2027.42/33481.
- ^ Ruffini G, Wendling F, Merlet I, Molaee-Ardekani B, Mekonnen A, Salvador R, et al. (May 2013). "Transcranial current brain stimulation (tCS): models and technologies". IEEE Transactions on Neural Systems and Rehabilitation Engineering. 21 (3): 333–345. CiteSeerX 10.1.1.352.4406. doi:10.1109/TNSRE.2012.2200046. PMID 22949089. S2CID 19615665.
- ^ Brunoni AR, Moffa AH, Fregni F, Palm U, Padberg F, Blumberger DM, et al. (June 2016). "Transcranial direct current stimulation for acute major depressive episodes: meta-analysis of individual patient data". The British Journal of Psychiatry. 208 (6): 522–531. doi:10.1192/bjp.bp.115.164715. PMC 4887722. PMID 27056623.
- ^ Brunoni AR, Moffa AH, Sampaio-Junior B, Borrione L, Moreno ML, Fernandes RA, et al. (June 2017). "Trial of Electrical Direct-Current Therapy versus Escitalopram for Depression". The New England Journal of Medicine. 376 (26): 2523–2533. doi:10.1056/nejmoa1612999. PMID 28657871.
- ^ a b Mondino M, Bennabi D, Poulet E, Galvao F, Brunelin J, Haffen E (May 2014). "Can transcranial direct current stimulation (tDCS) alleviate symptoms and improve cognition in psychiatric disorders?". The World Journal of Biological Psychiatry. 15 (4): 261–275. doi:10.3109/15622975.2013.876514. PMID 24447054. S2CID 33184990.
- ^ Coffman BA, Clark VP, Parasuraman R (January 2014). "Battery powered thought: enhancement of attention, learning, and memory in healthy adults using transcranial direct current stimulation". NeuroImage. 85 (Pt 3): 895–908. doi:10.1016/j.neuroimage.2013.07.083. PMID 23933040. S2CID 34389202.
- ^ Dedoncker J, Brunoni AR, Baeken C, Vanderhasselt MA (2016). "A Systematic Review and Meta-Analysis of the Effects of Transcranial Direct Current Stimulation (tDCS) Over the Dorsolateral Prefrontal Cortex in Healthy and Neuropsychiatric Samples: Influence of Stimulation Parameters". Brain Stimulation. 9 (4): 501–517. doi:10.1016/j.brs.2016.04.006. hdl:1854/LU-7206618. PMID 27160468. S2CID 98400.
- ^ Hill AT, Fitzgerald PB, Hoy KE (2016). "Effects of Anodal Transcranial Direct Current Stimulation on Working Memory: A Systematic Review and Meta-Analysis of Findings From Healthy and Neuropsychiatric Populations". Brain Stimulation. 9 (2): 197–208. doi:10.1016/j.brs.2015.10.006. PMID 26597929. S2CID 17093486.
- ^ Katsoulaki M, Kastrinis A, Tsekoura M (2017). "The Effects of Anodal Transcranial Direct Current Stimulation on Working Memory". GeNeDis 2016. Advances in Experimental Medicine and Biology. Vol. 987. pp. 283–289. doi:10.1007/978-3-319-57379-3_25. ISBN 978-3319573786. PMID 28971466.
- ^ a b Horvath JC, Forte JD, Carter O (January 2015). "Evidence that transcranial direct current stimulation (tDCS) generates little-to-no reliable neurophysiologic effect beyond MEP amplitude modulation in healthy human subjects: A systematic review". Neuropsychologia. 66: 213–236. doi:10.1016/j.neuropsychologia.2014.11.021. PMID 25448853. S2CID 27300914.
- ^ a b c Horvath JC, Forte JD, Carter O (2015). "Quantitative Review Finds No Evidence of Cognitive Effects in Healthy Populations From Single-session Transcranial Direct Current Stimulation (tDCS)". Brain Stimulation. 8 (3): 535–550. doi:10.1016/j.brs.2015.01.400. PMID 25701175. S2CID 19833275.
- ^ Chhatbar PY, Feng W (2015). "Data Synthesis in Meta-Analysis may Conclude Differently on Cognitive Effect From Transcranial Direct Current Stimulation". Brain Stimulation. 8 (5): 974–976. doi:10.1016/j.brs.2015.06.001. PMID 26115775. S2CID 1539586.
- ^ Horvath JC (2015). "New Quantitative Analyses Following Price & Hamilton's Critique do not Change Original Findings of Horvath et al". Brain Stimulation. 8 (3): 665–666. doi:10.1016/j.brs.2015.05.001. PMID 26050600. S2CID 38603410.
- ^ a b c Agarwal SM, Shivakumar V, Bose A, Subramaniam A, Nawani H, Chhabra H, et al. (December 2013). "Transcranial direct current stimulation in schizophrenia". Clinical Psychopharmacology and Neuroscience. 11 (3): 118–125. doi:10.9758/cpn.2013.11.3.118. PMC 3897759. PMID 24465247.
- ^ a b Pollock A, Farmer SE, Brady MC, Langhorne P, Mead GE, Mehrholz J, van Wijck F (November 2014). "Interventions for improving upper limb function after stroke". The Cochrane Database of Systematic Reviews. 2014 (11): CD010820. doi:10.1002/14651858.CD010820.pub2. PMC 6469541. PMID 25387001.
- ^ a b c d Feng WW, Bowden MG, Kautz S (Jan 2013). "Review of transcranial direct current stimulation in poststroke recovery". Topics in Stroke Rehabilitation. 20 (1): 68–77. doi:10.1310/tsr2001-68. PMID 23340073. S2CID 39688758.
- ^ Elsner B, Kugler J, Pohl M, Mehrholz J (May 2019). "Transcranial direct current stimulation (tDCS) for improving aphasia in adults with aphasia after stroke". The Cochrane Database of Systematic Reviews. 2019 (5): CD009760. doi:10.1002/14651858.CD009760.pub4. PMC 6528187. PMID 31111960.
- ^ a b de Aguiar V, Paolazzi CL, Miceli G (February 2015). "tDCS in post-stroke aphasia: the role of stimulation parameters, behavioral treatment and patient characteristics". Cortex; A Journal Devoted to the Study of the Nervous System and Behavior. 63: 296–316. doi:10.1016/j.cortex.2014.08.015. PMID 25460496. S2CID 22631885.
- ^ a b Buch ER, Santarnecchi E, Antal A, Born J, Celnik PA, Classen J, et al. (April 2017). "Effects of tDCS on motor learning and memory formation: A consensus and critical position paper" (PDF). Clinical Neurophysiology. 128 (4): 589–603. doi:10.1016/j.clinph.2017.01.004. PMID 28231477.
- ^ Boldt I, Eriks-Hoogland I, Brinkhof MW, de Bie R, Joggi D, von Elm E (November 2014). "Non-pharmacological interventions for chronic pain in people with spinal cord injury". The Cochrane Database of Systematic Reviews. 11 (11): CD009177. doi:10.1002/14651858.CD009177.pub2. PMC 11329868. PMID 25432061.
- ^ Pedron S, Monnin J, Haffen E, Sechter D, Van Waes V (March 2014). "Repeated transcranial direct current stimulation prevents abnormal behaviors associated with abstinence from chronic nicotine consumption". Neuropsychopharmacology. 39 (4): 981–988. doi:10.1038/npp.2013.298. PMC 3924532. PMID 24154668.
- ^ Jansen JM, Daams JG, Koeter MW, Veltman DJ, van den Brink W, Goudriaan AE (December 2013). "Effects of non-invasive neurostimulation on craving: a meta-analysis". Neuroscience and Biobehavioral Reviews. 37 (10 Pt 2): 2472–2480. doi:10.1016/j.neubiorev.2013.07.009. PMID 23916527. S2CID 1410164.
- ^ Lefaucheur JP, Antal A, Ayache SS, Benninger DH, Brunelin J, Cogiamanian F, et al. (January 2017). "Evidence-based guidelines on the therapeutic use of transcranial direct current stimulation (tDCS)". Clinical Neurophysiology. 128 (1): 56–92. doi:10.1016/j.clinph.2016.10.087. hdl:10067/1420300151162165141. PMID 27866120. S2CID 4837447.
- ^ Harty S, Robertson IH, Miniussi C, Sheehy OC, Devine CA, McCreery S, O'Connell RG (March 2014). "Transcranial direct current stimulation over right dorsolateral prefrontal cortex enhances error awareness in older age". The Journal of Neuroscience. 34 (10): 3646–3652. doi:10.1523/JNEUROSCI.5308-13.2014. PMC 6608991. PMID 24599463.
- ^ Grimaldi G, Argyropoulos GP, Bastian A, Cortes M, Davis NJ, Edwards DJ, et al. (February 2016). "Cerebellar Transcranial Direct Current Stimulation (ctDCS): A Novel Approach to Understanding Cerebellar Function in Health and Disease". The Neuroscientist. 22 (1): 83–97. doi:10.1177/1073858414559409. PMC 4712385. PMID 25406224.
- ^ van Dun K, Bodranghien FC, Mariën P, Manto MU (2016-01-01). "tDCS of the Cerebellum: Where Do We Stand in 2016? Technical Issues and Critical Review of the Literature". Frontiers in Human Neuroscience. 10: 199. doi:10.3389/fnhum.2016.00199. PMC 4862979. PMID 27242469.
- ^ van Dun K, Bodranghien F, Manto M, Mariën P (June 2017). "Targeting the Cerebellum by Noninvasive Neurostimulation: a Review". Cerebellum. 16 (3): 695–741. doi:10.1007/s12311-016-0840-7. PMID 28032321. S2CID 3999098.
- ^ a b Grimaldi G, Argyropoulos GP, Boehringer A, Celnik P, Edwards MJ, Ferrucci R, et al. (2014). "Non-invasive cerebellar stimulation--a consensus paper" (PDF). Cerebellum. 13 (1): 121–138. doi:10.1007/s12311-013-0514-7
- ^ Siebner HR, Hartwigsen G, Kassuba T, Rothwell JC (2009). "How does transcranial magnetic stimulation modify neuronal activity in the brain? Implications for studies of cognition". Cortex; A Journal Devoted to the Study of the Nervous System and Behavior. 45 (9): 1035–1042. doi:10.1016/j.cortex.2009.02.007
- ^ a b Sparing R, Mottaghy FM (2008). "Noninvasive brain stimulation with transcranial magnetic or direct current stimulation (TMS/tDCS)-From insights into human memory to therapy of its dysfunction". Methods. 44 (4): 329–337. doi:10.1016/j.ymeth.2007.02.001
- ^ "FDA Executive Summary – Petitions to Request Change in Classification for Cranial Electrotherapy Stimulators" (PDF). Food and Drug Administration.
