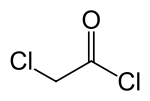
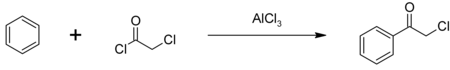Chloroacetyl chloride
| |

| |
| Names | |
|---|---|
| Preferred IUPAC name
Chloroacetyl chloride | |
| Other names
2-Chloroacetyl chloride
Chloroacetic acid chloride Chloroacetic chloride Monochloroacetyl chloride | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.001.065 |
| EC Number |
|
| KEGG | |
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| UN number | 1752 |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C2H2Cl2O | |
| Molar mass | 112.94 g·mol−1 |
| Appearance | Colorless to yellow liquid |
| Density | 1.42 g/mL |
| Melting point | −22 °C (−8 °F; 251 K) |
| Boiling point | 106 °C (223 °F; 379 K) |
| Reacts | |
| Vapor pressure | 19 mmHg (20°C)[1] |
| Hazards | |
| GHS labelling: | |
   
| |
| Danger | |
| H301, H311, H314, H331, H372, H400 | |
| P260, P261, P264, P270, P271, P273, P280, P301+P310, P301+P330+P331, P302+P352, P303+P361+P353, P304+P340, P305+P351+P338, P310, P311, P312, P314, P321, P322, P330, P361, P363, P391, P403+P233, P405, P501 | |
| NFPA 704 (fire diamond) | |
| Flash point | noncombustible[1] |
| NIOSH (US health exposure limits): | |
PEL (Permissible)
|
none[1] |
REL (Recommended)
|
TWA 0.05 ppm (0.2 mg/m3)[1] |
IDLH (Immediate danger)
|
N.D.[1] |
| Safety data sheet (SDS) | Oxford MSDS |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Chloroacetyl chloride is a chlorinated acyl chloride. It is a bifunctional compound, making it a useful building block chemical.
Production
[edit]Industrially, it is produced by the carbonylation of methylene chloride, oxidation of vinylidene chloride, or the addition of chlorine to ketene.[3] It may be prepared from chloroacetic acid and thionyl chloride, phosphorus pentachloride, or phosgene.
Reactions
[edit]Chloroacetyl chloride is bifunctional—the acyl chloride easily forms esters[4] and amides, while the other end of the molecule is able to form other linkages, e.g. with amines. The use of chloroacetyl chloride in the synthesis of lidocaine is illustrative:[5]
Applications
[edit]The major use of chloroacetyl chloride is as an intermediate in the production of herbicides in the chloroacetanilide family including metolachlor, acetochlor, alachlor and butachlor; an estimated 100 million pounds are used annually. Some chloroacetyl chloride is also used to produce phenacyl chloride, another chemical intermediate, also used as a tear gas.[3] Phenacyl chloride is synthesized in a Friedel-Crafts acylation of benzene, with an aluminium chloride catalyst:[6]
With anisole, it is used for the synthesis of venlafaxine.
Safety
[edit]Like other acyl chlorides, reaction with other protic compounds such as amines, alcohols, and water generates hydrochloric acid, making it a lachrymator.
There is no regulated permissible exposure limit set by the Occupational Safety and Health Administration. However, the National Institute for Occupational Safety and Health has set a recommended exposure limit at 0.05 ppm over an eight-hour work day.[7]
References
[edit]- ^ a b c d e NIOSH Pocket Guide to Chemical Hazards. "#0120". National Institute for Occupational Safety and Health (NIOSH).
- ^ "OSHA Occupational Chemical Database". Occupational Safety and Health Administration (OSHA).
- ^ a b Paul R. Worsham (1993). "15. Halogenated Derivatives" (Google Books excerpt). In Zoeller, Joseph R.; Agreda, V. H. (eds.). Acetic acid and its derivatives. New York: M. Dekker. pp. 288–298. ISBN 0-8247-8792-7.
- ^ Robert H. Baker and Frederick G. Bordwell (1955). "tert-Butyl acetate". Organic Syntheses; Collected Volumes, vol. 3.
- ^ T. J. Reilly (1999). "The Preparation of Lidocaine". J. Chem. Educ. 76 (11): 1557. Bibcode:1999JChEd..76.1557R. doi:10.1021/ed076p1557.
- ^ Nathan Levin and Walter H. Hartung (1955). "ω-Chloroisonitrosoacetophenone". Organic Syntheses; Collected Volumes, vol. 3, p. 191.
- ^ "NIOSH Pocket Guide to Chemical Hazards". Centers for Disease Control and Prevention. 2011.