Agricultural chemistry
Agricultural chemistry is the chemistry, especially organic chemistry and biochemistry, as they relate to agriculture. Agricultural chemistry embraces the structures and chemical reactions relevant in the production, protection, and use of crops and livestock. Its applied science and technology aspects are directed towards increasing yields and improving quality, which comes with multiple advantages and disadvantages.[1]
Agricultural and environmental chemistry
[edit]This aspect of agricultural chemistry deals with the role of molecular chemistry in agriculture as well as the negative consequences.
Plant Biochemistry
[edit]Plant biochemistry encompasses the chemical reactions that occur within plants. In principle, knowledge at a molecular level informs technologies for providing food. Particular focus is on the biochemical differences between plants and other organisms as well as the differences within the plant kingdom, such as dicotyledons vs monocotyledons, gymnosperms vs angiosperms, C2- vs C4-fixers, etc.
Pesticides
[edit]
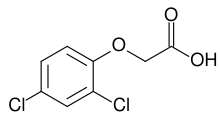
Chemical materials developed to assist in the production of food, feed, and fiber include herbicides, insecticides, fungicides,[2] and other pesticides. Pesticides are chemicals that play an important role in increasing crop yield and mitigating crop losses.[3] These work to keep insects and other animals away from crops to allow them to grow undisturbed, effectively regulating pests and diseases.
Disadvantages of pesticides include contamination of the ground and water (see persistent organic pollutants). They may be toxic to non-target species, including birds, fish,[4] pollinators, [5] as well as the farmworkers themselves.
Soil Chemistry
[edit]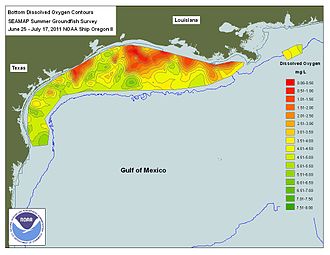
Agricultural chemistry often aims at preserving or increasing the fertility of soil with the goals of maintaining or improving the agricultural yield and improving the quality of the crop. Soils are analyzed with attention to the inorganic matter (minerals), which comprise most of the mass of dry soil, and organic matter, which consists of living organisms, their degradation products, humic acids and fulvic acids. [8]
Fertilizers are a major consideration. While organic fertilizers are time-honored, their use has largely been displaced by chemicals produced from mining (phosphate rock) and the Haber-Bosch process. The use of these materials dramatically increased the rate at which crops are produced, which is able to support the growing human population. Common fertilizers include urea, ammonium sulphate, diammonium phosphate, and calcium ammonium phosphate.[9][10]
Biofuels and bio-derived materials
[edit]
Agricultural chemistry encompases the science and technology of producing not only edible crops, but feedstocks for fuels ("biofuels") and materials. Ethanol fuel obtained by fermentation of sugars. Biodiesel is derived from fats, both animal- and plant-derived. Methane can be recovered from manure and other ag wastes by microbial action.[11][12] Lignocellulose is a promising precursor to new materials.[13]
Biotechnology
[edit]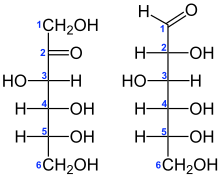
Biocatalysis is used to produce a number of food products. More than five biilion tons of high fructose corn syrup are produced annually by the action of the immobilized enzyme glucose isomerase of corn-derived glucose. Emerging technologies are numerous, including enzymes for clarifying or debittering of fruit juices.[14]
A variety of potentially useful chemicals are obtained by engineered plants. Bioremediation is a green route to biodegradation.
GMOs
[edit]Genetically Modified Organisms (GMO's) are plants or living things that have been altered at a genomic level by scientists to improve the organisms characteristics. These characteristics include providing new vaccines for humans, increasing nutrients supplies, and creating unique plastics.[15] They may also be able to grow in climates that are typically not suitable for the original organism to grow in.[15] Examples of GMO's include virus resistant tobacco and squash, delayed ripening tomatoes, and herbicide resistant soybeans.[15]
GMO's came with an increased interest in using biotechnology to produce fertilizer and pesticides. Due to an increased market interest in biotechnology in the 1970s, there was more technology and infrastructure developed, a decreased cost, and an advance in research. Since the early 1980s, genetically-modified crops have been incorporated. Increased biotechnological work calls for the union of biology and chemistry to produce improved crops, a main reason behind this being the increasing amount of food needed to feed a growing population.[16]
That being said, concerns with GMO's include potential antibiotic resistance from eating a GMO.[15] There are also concerns about the long term effects on the human body since many GMO's were recently developed.[15]
Much controversy surrounds GMO's. In the United States, all foods containing GMO's must be labeled as such.[17]
Omics
[edit]Particularly relevant is proteomics as protein (nutrition) guides much of agriculture.
See also
[edit]Notes and references
[edit]- ^ "Scope, Journal of Agricultural Chemistry".
- ^ Dreikorn, Barry A.; Owen, W. John (2000). "Fungicides, Agricultural". Kirk-Othmer Encyclopedia of Chemical Technology. doi:10.1002/0471238961.0621140704180509.a01. ISBN 978-0-471-48494-3.
- ^ al-Saleh, I. A. (1994). "Pesticides: a review article". Journal of Environmental Pathology, Toxicology and Oncology. 13 (3): 151–161. PMID 7722882. INIST 3483983.
- ^ Aktar, Wasim; Sengupta, Dwaipayan; Chowdhury, Ashim (March 2009). "Impact of pesticides use in agriculture: their benefits and hazards". Interdisciplinary Toxicology. 2 (1): 1–12. doi:10.2478/v10102-009-0001-7. PMC 2984095. PMID 21217838.
- ^ Bomgardner, Melody; Erickson, Britt (13 January 2020). "Food brands and retailers will scrutinize pesticides". C&EN Global Enterprise. 98 (2): 33. doi:10.1021/cen-09802-cover8.
- ^ "NOAA: Gulf of Mexico 'Dead Zone' Predictions Feature Uncertainty". U.S. Geological Survey (USGS). June 21, 2012. Archived from the original on 2016-04-11. Retrieved June 23, 2012.
- ^ "What is hypoxia?". Louisiana Universities Marine Consortium (LUMCON). Archived from the original on June 12, 2013. Retrieved May 18, 2013.
- ^ Arai, Yuji (2016). "Soil Chemistry". Kirk-Othmer Encyclopedia of Chemical Technology. pp. 1–37. doi:10.1002/0471238961.koe00021. ISBN 978-0-471-48494-3.
- ^ Rouwenhorst, K.H.R.; Elishav, O.; Mosevitzky Lis, B.; Grader, G.S.; Mounaïm-Rousselle, C.; Roldan, A.; Valera-Medina, A. (2021). "Future Trends" (PDF). Techno-Economic Challenges of Green Ammonia as an Energy Vector. pp. 303–319. doi:10.1016/B978-0-12-820560-0.00013-8. ISBN 978-0-12-820560-0. S2CID 243358894.
- ^ Leghari, Shah Jahan; Wahocho, Niaz Ahmed; Laghari, Ghulam Mustafa; HafeezLaghari, Abdul; MustafaBhabhan, Ghulam; HussainTalpur, Khalid; Bhutto, Tofique Ahmed; Wahocho, Safdar Ali; Lashari, Ayaz Ahmed (September 2016). "Role of nitrogen for plant growth and development: a review". Advances in Environmental Biology. 10 (9): 209–219. Gale A472372583.
- ^ Murzin, Dmitry Yu.; Mäki-Arvela, Päivi; Simakova, Irina L. (2012). "Triglycerides and Oils for Biofuels". Kirk-Othmer Encyclopedia of Chemical Technology. pp. 1–14. doi:10.1002/0471238961.trigmurz.a01. ISBN 978-0-471-48494-3.
- ^ Paisley, Mark A. (2003). "Biomass Energy". Kirk-Othmer Encyclopedia of Chemical Technology. doi:10.1002/0471238961.0621051211120119.a01.pub2. ISBN 978-0-471-48494-3.
- ^ Upton, Brianna M.; Kasko, Andrea M. (2016). "Strategies for the Conversion of Lignin to High-Value Polymeric Materials: Review and Perspective". Chemical Reviews. 116 (4): 2275–2306. doi:10.1021/acs.chemrev.5b00345.
- ^ Dicosimo, Robert; McAuliffe, Joseph; Poulose, Ayrookaran J.; Bohlmann, Gregory (2013). "Industrial Use of Immobilized Enzymes". Chemical Society Reviews. 42 (15): 6437. doi:10.1039/c3cs35506c. PMID 23436023.
- ^ a b c d e Bawa, A. S.; Anilakumar, K. R. (December 2013). "Genetically modified foods: safety, risks and public concerns—a review". Journal of Food Science and Technology. 50 (6): 1035–1046. doi:10.1007/s13197-012-0899-1. PMC 3791249. PMID 24426015.
- ^ Meadows-Smith, Marcus; Meadows-Smith, Holly (3 July 2017). "Perspectives: Chemistry seeks its new level in agtech". C&EN Global Enterprise. 95 (27): 22–23. doi:10.1021/cen-09527-scitech2.
- ^ Erickson, Britt (18 July 2016). "House clears GMO food labeling bill". C&EN Global Enterprise. 94 (29): 16. doi:10.1021/cen-09429-notw11.
