Bioremediation
| Part of a series on |
| Pollution |
|---|
 |
Bioremediation broadly refers to any process wherein a biological system (typically bacteria, microalgae, fungi in mycoremediation, and plants in phytoremediation), living or dead, is employed for removing environmental pollutants from air, water, soil, flue gasses, industrial effluents etc., in natural or artificial settings.[1] The natural ability of organisms to adsorb, accumulate, and degrade common and emerging pollutants has attracted the use of biological resources in treatment of contaminated environment.[1] In comparison to conventional physicochemical treatment methods bioremediation may offer advantages as it aims to be sustainable, eco-friendly, cheap, and scalable.[1]
Most bioremediation is inadvertent, involving native organisms. Research on bioremediation is heavily focused on stimulating the process by inoculation of a polluted site with organisms or supplying nutrients to promote their growth. Environmental remediation is an alternative to bioremediation.[2]
While organic pollutants are susceptible to biodegradation, heavy metals cannot be degraded, but rather oxidized or reduced. Typical bioremediations involves oxidations.[3][4] Oxidations enhance the water-solubility of organic compounds and their susceptibility to further degradation by further oxidation and hydrolysis. Ultimately biodegradation converts hydrocarbons to carbon dioxide and water.[5] For heavy metals, bioremediation offers few solutions. Metal-containing pollutant can be removed, at least partially, with varying bioremediation techniques.[6] The main challenge to bioremediations is rate: the processes are slow.[7]
Bioremediation techniques can be classified as (i) in situ techniques, which treat polluted sites directly, vs (ii) ex situ techniques which are applied to excavated materials.[8] In both these approaches, additional nutrients, vitamins, minerals, and pH buffers are added to enhance the growth and metabolism of the microorganisms. In some cases, specialized microbial cultures are added (biostimulation). Some examples of bioremediation related technologies are phytoremediation, bioventing, bioattenuation, biosparging, composting (biopiles and windrows), and landfarming. Other remediation techniques include thermal desorption, vitrification, air stripping, bioleaching, rhizofiltration, and soil washing. Biological treatment, bioremediation, is a similar approach used to treat wastes including wastewater, industrial waste and solid waste. The end goal of bioremediation is to remove harmful compounds to improve soil and water quality.[9]
In situ techniques
[edit]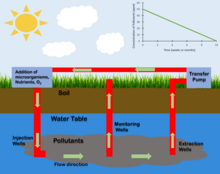
Bioventing
[edit]Bioventing is a process that increases the oxygen or air flow into the unsaturated zone of the soil, this in turn increases the rate of natural in situ degradation of the targeted hydrocarbon contaminant.[11] Bioventing, an aerobic bioremediation, is the most common form of oxidative bioremediation process where oxygen is provided as the electron acceptor for oxidation of petroleum, polyaromatic hydrocarbons (PAHs), phenols, and other reduced pollutants. Oxygen is generally the preferred electron acceptor because of the higher energy yield and because oxygen is required for some enzyme systems to initiate the degradation process.[7] Microorganisms can degrade a wide variety of hydrocarbons, including components of gasoline, kerosene, diesel, and jet fuel. Under ideal aerobic conditions, the biodegradation rates of the low- to moderate-weight aliphatic, alicyclic, and aromatic compounds can be very high. As molecular weight of the compound increases, the resistance to biodegradation increases simultaneously.[7] This results in higher contaminated volatile compounds due to their high molecular weight and an increased difficulty to remove from the environment.
Most bioremediation processes involve oxidation-reduction reactions where either an electron acceptor (commonly oxygen) is added to stimulate oxidation of a reduced pollutant (e.g. hydrocarbons) or an electron donor (commonly an organic substrate) is added to reduce oxidized pollutants (nitrate, perchlorate, oxidized metals, chlorinated solvents, explosives and propellants).[5] In both these approaches, additional nutrients, vitamins, minerals, and pH buffers may be added to optimize conditions for the microorganisms. In some cases, specialized microbial cultures are added (bioaugmentation) to further enhance biodegradation.
Approaches for oxygen addition below the water table include recirculating aerated water through the treatment zone, addition of pure oxygen or peroxides, and air sparging.[12] Recirculation systems typically consist of a combination of injection wells or galleries and one or more recovery wells where the extracted groundwater is treated, oxygenated, amended with nutrients and re-injected.[13] However, the amount of oxygen that can be provided by this method is limited by the low solubility of oxygen in water (8 to 10 mg/L for water in equilibrium with air at typical temperatures). Greater amounts of oxygen can be provided by contacting the water with pure oxygen or addition of hydrogen peroxide (H2O2) to the water. In some cases, slurries of solid calcium or magnesium peroxide are injected under pressure through soil borings. These solid peroxides react with water releasing H2O2 which then decomposes releasing oxygen. Air sparging involves the injection of air under pressure below the water table. The air injection pressure must be great enough to overcome the hydrostatic pressure of the water and resistance to air flow through the soil.[12][13]
Biostimulation
[edit]
Bioremediation can be carried out by bacteria that are naturally present. In biostimulation, the population of these helpful bacteria can be increased by adding nutrients.[6][15]
Bacteria can in principle be used to degrade hydrocarbons.[16][17] Specific to marine oil spills, nitrogen and phosphorus have been key nutrients in biodegradation.[18] The bioremediation of hydrocarbons suffers from low rates.
Bioremediation can involve the action of microbial consortium. Within the consortium, the product of one species could be the substrate for another species.[19]
Anaerobic bioremediation can in principle be employed to treat a range of oxidized contaminants including chlorinated ethylenes (PCE, TCE, DCE, VC), chlorinated ethanes (TCA, DCA), chloromethanes (CT, CF), chlorinated cyclic hydrocarbons, various energetics (e.g., perchlorate,[20] RDX, TNT), and nitrate.[6] This process involves the addition of an electron donor to: 1) deplete background electron acceptors including oxygen, nitrate, oxidized iron and manganese and sulfate; and 2) stimulate the biological and/or chemical reduction of the oxidized pollutants. The choice of substrate and the method of injection depend on the contaminant type and distribution in the aquifer, hydrogeology, and remediation objectives. Substrate can be added using conventional well installations, by direct-push technology, or by excavation and backfill such as permeable reactive barriers (PRB) or biowalls.[21] Slow-release products composed of edible oils or solid substrates tend to stay in place for an extended treatment period. Soluble substrates or soluble fermentation products of slow-release substrates can potentially migrate via advection and diffusion, providing broader but shorter-lived treatment zones. The added organic substrates are first fermented to hydrogen (H2) and volatile fatty acids (VFAs). The VFAs, including acetate, lactate, propionate and butyrate, provide carbon and energy for bacterial metabolism.[6][5]
Bioattenuation
[edit]During bioattenuation, biodegradation occurs naturally with the addition of nutrients or bacteria. The indigenous microbes present will determine the metabolic activity and act as a natural attenuation.[22] While there is no anthropogenic involvement in bioattenuation, the contaminated site must still be monitored.[22]
Biosparging
[edit]Biosparging is the process of groundwater remediation as oxygen, and possible nutrients, is injected. When oxygen is injected, indigenous bacteria are stimulated to increase rate of degradation.[23] However, biosparging focuses on saturated contaminated zones, specifically related to ground water remediation.[24]
UNICEF, power producers, bulk water suppliers, and local governments are early adopters of low cost bioremediation, such as aerobic bacteria tablets which are simply dropped into water.[25]
Ex situ techniques
[edit]Biopiles
[edit]Biopiles, similar to bioventing, are used to remove petroleum pollutants by introducing aerobic hydrocarbons to contaminated soils. However, the soil is excavated and piled with an aeration system. This aeration system enhances microbial activity by introducing oxygen under positive pressure or removes oxygen under negative pressure.[26]
Windrows
[edit]
Windrow systems are similar to compost techniques where soil is periodically turned in order to enhance aeration.[28] This periodic turning also allows contaminants present in the soil to be uniformly distributed which accelerates the process of bioremediation.[29]
Landfarming
[edit]Landfarming, or land treatment, is a method commonly used for sludge spills. This method disperses contaminated soil and aerates the soil by cyclically rotating.[30] This process is an above land application and contaminated soils are required to be shallow in order for microbial activity to be stimulated. However, if the contamination is deeper than 5 feet, then the soil is required to be excavated to above ground.[13] While it is an ex situ technique, it can also be considered an in situ technique as Landfarming can be performed at the site of contamination.[31]
In situ vs. Ex situ
[edit]Ex situ techniques are often more expensive because of excavation and transportation costs to the treatment facility, while in situ techniques are performed at the site of contamination so they only have installation costs. While there is less cost there is also less of an ability to determine the scale and spread of the pollutant. The pollutant ultimately determines which bioremediation method to use. The depth and spread of the pollutantare other important factors.[32]
Heavy metals
[edit]Heavy metals are introduced into the environment by both anthropogenic activities and natural factors.[6] Anthropogenic activities include industrial emissions, electronic waste, and mining. Natural factors include mineral weathering, soil erosion, and forest fires.[6] Heavy metals including cadmium, chromium, lead and uranium are unlike organic compounds and cannot be biodegraded. However, bioremediation processes can potentially be used to minimize the mobility of these material in the subsurface, lowering the potential for human and environmental exposure.[33] Heavy metals from these factors are predominantly present in water sources due to runoff where it is uptake by marine fauna and flora.[6]
Hexavalent chromium (Cr[VI]) and uranium (U[VI]) can be reduced to less mobile and/or less toxic forms (e.g., Cr[III], U[IV]). Similarly, reduction of sulfate to sulfide (sulfidogenesis) can be used to immobilize certain metals (e.g., zinc, cadmium).
The mobility of certain metals including chromium (Cr) and uranium (U) varies depending on the oxidation state of the material.[34] Microorganisms can be used to lower the toxicity and mobility of chromium by reducing hexavalent chromium, Cr(VI) to trivalent Cr(III).[35] Reduction of the more mobile U(VI) species affords the less mobile U(IV) derivatives.[36][37] Microorganisms are used in this process because the reduction rate of these metals is often slow in the absence of microbial interactions[38] Research is also underway to develop methods to remove metals from water by enhancing the sorption of the metal to cell walls.[38] This approach has been evaluated for treatment of cadmium,[39] chromium,[40] and lead.[41] Genetically modified bacteria has also been explored for use in sequestration of Arsenic.[42] Phytoextraction processes concentrate contaminants in the biomass for subsequent removal.
Metal extractions can in principle be performed in situ or ex situ where in situ is preferred since it is less expensive to excavate the substrate.[43]
Bioremediation is not specific to metals. In 2010 there was a massive oil spill in the Gulf of Mexico. Populations of bacteria and archaea were used to rejuvenate the coast after the oil spill. These microorganisms over time have developed metabolic networks that can utilize hydrocarbons such as oil and petroleum as a source of carbon and energy.[44] Microbial bioremediation is a very effective modern technique for restoring natural systems by removing toxins from the environment.
Pesticides
[edit]Of the many ways to deal with pesticide contamination, bioremediation promises to be more effective.[45] Many sites around the world are contaminated with agrichemicals. [46] These agrichemicals often resist biodegradation, by design.[47][48] Harming all manners of organic life with long term health issues such as cancer, rashes, blindness, paralysis, and mental illness.[47] An example is Lindane which was a commonly used insecticide in the 20th century. Long time exposure poses a serious threat to humans and the surrounding ecosystem. Lindane reduces the potential of beneficial bacteria in the soil such as nitrogen fixation cyanobacteria. As well as causing central nervous system issues in smaller mammals such as seizures, dizziness, and even death. What makes it so harmful to these organisms is how quickly distributed it gets through the brain and fatty tissues. While Lindane has been mostly limited to specific use, it is still produced and used around the world.[49]
Actinobacteria has been a promising candidate in situ technique specifically for removing pesticides. When certain strains of Actinobacteria have been grouped together, their efficiency in degrading pesticides has enhanced. As well as being a reusable technique that strengthens through further use by limiting the migration space of these cells to target specific areas and not fully consume their cleansing abilities. Despite encouraging results, Actinobacteria has only been used in controlled lab settings and will need further development in finding the cost effectiveness and scalability of use.[50]
Limitations of bioremediation
[edit]Bioremediation can be used to mineralize organic pollutants, to partially transform the pollutants, or alter their mobility. Heavy metals and radionuclides generally cannot be biodegraded, but can be bio-transformed to less mobile forms.[51][52][53] In some cases, microbes do not fully mineralize the pollutant, potentially producing a more toxic compound.[53] For example, under anaerobic conditions, the reductive dehalogenation of TCE may produce dichloroethylene (DCE) and vinyl chloride (VC), which are suspected or known carcinogens.[51] However, the microorganism Dehalococcoides can further reduce DCE and VC to the non-toxic product ethene.[54] The molecular pathways for bioremediation are of considerable interest.[51] In addition, knowing these pathways will help develop new technologies that can deal with sites that have uneven distributions of a mixture of contaminants.[23]
Biodegradation requires microbial population with the metabolic capacity to degrade the pollutant.[23][52] The biological processes used by these microbes are highly specific, therefore, many environmental factors must be taken into account and regulated as well.[23][51] It can be difficult to extrapolate the results from the small-scale test studies into big field operations.[23] In many cases, bioremediation takes more time than other alternatives such as land filling and incineration.[23][51] Another example is bioventing, which is inexpensive to bioremediate contaminated sites, however, this process is extensive and can take a few years to decontaminate a site.[55]>
Another major drawback is finding the right species to perform bioremediation. In order to prevent the introduction and spreading of an invasive species to the ecosystem, an indigenous species is needed. As well as a species plentiful enough to clean the whole site without exhausting the population. Finally the species should be resilient enough to withstand the environmental conditions.[56] These specific criteria may make it difficult to perform bioremediation on a contaminated site.
In agricultural industries, the use of pesticides is a top factor in direct soil contamination and runoff water contamination. The limitation or remediation of pesticides is the low bioavailability.[57] Altering the pH and temperature of the contaminated soil is a resolution to increase bioavailability which, in turn, increased degradation of harmful compounds.[57]
The compound acrylonitrile is commonly produced in industrial setting but adversely contaminates soils. Microorganisms containing nitrile hydratases (NHase) degraded harmful acrylonitrile compounds into non-polluting substances.[58]
Since the experience with harmful contaminants are limited, laboratory practices are required to evaluate effectiveness, treatment designs, and estimate treatment times.[55] Bioremediation processes may take several months to several years depending on the size of the contaminated area.[59]
Genetic engineering
[edit]The use of genetic engineering to create organisms specifically designed for bioremediation is under preliminary research.[60] Two category of genes can be inserted in the organism: degradative genes, which encode proteins required for the degradation of pollutants, and reporter genes, which encode proteins able to monitor pollution levels.[61] Numerous members of Pseudomonas have been modified with the lux gene for the detection of the polyaromatic hydrocarbon naphthalene. A field test for the release of the modified organism has been successful on a moderately large scale.[62]
There are concerns surrounding release and containment of genetically modified organisms into the environment due to the potential of horizontal gene transfer.[63] Genetically modified organisms are classified and controlled under the Toxic Substances Control Act of 1976 under United States Environmental Protection Agency.[64] Measures have been created to address these concerns. Organisms can be modified such that they can only survive and grow under specific sets of environmental conditions.[63] In addition, the tracking of modified organisms can be made easier with the insertion of bioluminescence genes for visual identification.[65]
Genetically modified organisms have been created to treat oil spills and break down certain plastics (PET).[66]
Additive manufacturing
[edit]Additive manufacturing technologies such as bioprinting offer distinctive benefits that can be leveraged in bioremediation to develop structures with characteristics tailored to biological systems and environmental cleanup needs, and even though the adoption of this technology in bioremediation is in its early stages, the area is seeing massive growth.[67]
See also
[edit]- Bioremediation of radioactive waste
- Biosurfactant
- Chelation
- Dutch pollutant standards
- Folkewall
- In situ chemical oxidation
- In situ chemical reduction
- List of environment topics
- Mega Borg Oil Spill
- Microbial biodegradation
- Mycoremediation
- Mycorrhizal bioremediation
- Pleurotus
- Phytoremediation
- Pseudomonas putida (used for degrading oil)
- Restoration ecology
- Xenocatabolism
References
[edit]- ^ a b c Yuvraj (2022). "Microalgal Bioremediation: A Clean and Sustainable Approach for Controlling Environmental Pollution". Innovations in Environmental Biotechnology. Vol. 1. Singapore: Springer Singapore. pp. 305–318. doi:10.1007/978-981-16-4445-0_13. ISBN 978-981-16-4445-0.
- ^ "Green Remediation Best Management Practices: Sites with Leaking Underground Storage Tank Systems. EPA 542-F-11-008" (PDF). EPA. June 2011.
- ^ Duran N, Esposito E (2022). "Potential Applications of Oxidative Enzymes and Phenoloxidase-like Compounds in Wastewater and Soil Treatment: A Review". Applied Catalysis B: Environmental. 1 (2): 305–318. doi:10.1016/S0926-3373(00)00168-5.
- ^ Singh N, Kumar A, Sharma B (2019). "Role of Fungal Enzymes for Bioremediation of Hazardous Chemicals". Recent Advancement in White Biotechnology Through Fungi. Fungal Biology. Vol. 3. Cham: Springer International Publishing. pp. 237–256. doi:10.1007/978-3-030-25506-0_9. ISBN 978-3-030-25506-0. S2CID 210291135.
- ^ a b c Introduction to In Situ Bioremediation of Groundwater (PDF). US Environmental Protection Agency. 2013. p. 30.
- ^ a b c d e f g h Kapahi M, Sachdeva S (December 2019). "Bioremediation Options for Heavy Metal Pollution". Journal of Health and Pollution. 9 (24): 191203. doi:10.5696/2156-9614-9.24.191203. PMC 6905138. PMID 31893164.
- ^ a b c Mirza Hasanuzzaman, Majeti Narasimha Vara Prasad (2020). Handbook of Bioremediation. Academic Press. doi:10.1016/C2018-0-05109-9. ISBN 978-0-12-819382-2. S2CID 127409446.
- ^ Kensa VM (2011). "Bioremediation - An Overview". I Control Pollution. 27 (2): 161–168. ISSN 0970-2083.
- ^ a b Canak S, Berezljev L, Borojevic K, Asotic J, Ketin S (2019). "Bioremediation and "green chemistry"". Fresenius Environmental Bulletin. 28 (4): 3056–3064.
- ^ Jørgensen KS (2007). "In Situ Bioremediation". Advances in Applied Microbiology. 61. Academic Press: 285–305. doi:10.1016/S0065-2164(06)61008-3. ISBN 978-0-12-002663-0. PMID 17448793.
- ^ García Frutos FJ, Escolano O, García S, Babín M, Fernández MD (November 2010). "Bioventing remediation and ecotoxicity evaluation of phenanthrene-contaminated soil". Journal of Hazardous Materials. 183 (1–3): 806–13. Bibcode:2010JHzM..183..806F. doi:10.1016/j.jhazmat.2010.07.098. PMID 20800967.
- ^ a b Leeson A (2002). Air Sparging Design Paradigm (PDF). Columbus OH: Battelle. Archived from the original on June 20, 2017.
- ^ a b c "How To Evaluate Alternative Cleanup Technologies For Underground Storage Tank Sites. A Guide For Corrective Action Plan Reviewers" (PDF). EPA 510-B-17-003. United States Environmental Protection Agency (USEPA). 2017.
- ^ a b Mora RH, Macbeth TW, MacHarg T, Gundarlahalli J, Holbrook H, Schiff P (2008). "Enhanced bioremediation using whey powder for a trichloroethene plume in a high-sulfate, fractured granitic aquifer". Remediation Journal. 18 (3): 7–30. Bibcode:2008RemJ...18c...7M. doi:10.1002/rem.20168. ISSN 1520-6831.
- ^ Kalantary RR, Mohseni-Bandpi A, Esrafili A, Nasseri S, Ashmagh FR, Jorfi S, et al. (December 2014). "Effectiveness of biostimulation through nutrient content on the bioremediation of phenanthrene contaminated soil". Journal of Environmental Health Science & Engineering. 12 (1): 143. Bibcode:2014JEHSE..12..143K. doi:10.1186/s40201-014-0143-1. PMC 4301987. PMID 25610635.
- ^ Lee DW, Lee H, Lee AH, Kwon BO, Khim JS, Yim UH, et al. (March 2018). "Microbial community composition and PAHs removal potential of indigenous bacteria in oil contaminated sediment of Taean coast, Korea". Environmental Pollution. 234: 503–512. Bibcode:2018EPoll.234..503L. doi:10.1016/j.envpol.2017.11.097. PMID 29216488.
- ^ Chen Q, Bao B, Li Y, Liu M, Zhu B, Mu J, et al. (2020). "Effects of marine oil pollution on microbial diversity in coastal waters and stimulating indigenous microorganism bioremediation with nutrients". Regional Studies in Marine Science. 39: 101395. Bibcode:2020RSMS...3901395C. doi:10.1016/j.rsma.2020.101395. ISSN 2352-4855. S2CID 225285497.
- ^ Varjani SJ, Upasani VN (2017). "A new look on factors affecting microbial degradation of petroleum hydrocarbon pollutants". International Biodeterioration & Biodegradation. 120: 71–83. Bibcode:2017IBiBi.120...71V. doi:10.1016/j.ibiod.2017.02.006. ISSN 0964-8305.
- ^ Paniagua-Michel J, Fathepure BZ (2018). "Microbial Consortia and Biodegradation of Petroleum Hydrocarbons in Marine Environments". In Kumar V, Kumar M, Prasad R (eds.). Microbial Action on Hydrocarbons. Singapore: Springer Singapore. pp. 1–20. doi:10.1007/978-981-13-1840-5_1. ISBN 978-981-13-1839-9.
- ^ Coates JD, Jackson WA (2008). "Principles of Perchlorate Treatment". In Stroo H, Ward CH (eds.). In Situ Bioremediation of Perchlorate in Groundwater. SERDP/ESTCP Environmental Remediation Technology. New York: Springer. pp. 29–53. doi:10.1007/978-0-387-84921-8_3. ISBN 978-0-387-84921-8.
- ^ Gavaskar A, Gupta N, Sass B, Janosy R, Hicks J (March 2000). "Design guidance for application of permeable reactive barriers for groundwater remediation". Columbus OH: Battelle.
- ^ a b Ying GG (2018). "Chapter 14 - Remediation and Mitigation Strategies". Integrated Analytical Approaches for Pesticide Management. Academic Press. pp. 207–217. doi:10.1016/b978-0-12-816155-5.00014-2. ISBN 978-0-12-816155-5.
- ^ a b c d e f Vidali M (2001). "Bioremediation. An overview" (PDF). Pure and Applied Chemistry. 73 (7): 1163–72. doi:10.1351/pac200173071163. S2CID 18507182.
- ^ Johnson PC, Johnson RL, Bruce CL, Leeson A (2001). "Advances in In Situ Air Sparging/Biosparging". Bioremediation Journal. 5 (4): 251–266. Bibcode:2001BiorJ...5..251J. doi:10.1080/20018891079311. ISSN 1088-9868. S2CID 131393543.
- ^ "Ageing infrastructure gets bio boost". CAXTON. June 2022.
- ^ Chen R, Zhou Y (April 2021). "Measure microbial activity driven oxygen transfer in membrane aerated biofilm reactor from supply side". Environmental Research. 195: 110845. Bibcode:2021ER....19510845C. doi:10.1016/j.envres.2021.110845. PMID 33549616. S2CID 231867176.
- ^ a b Waters JM, Lambert C, Reid D, Shaw R (2002). Redevelopment of the former Shell Haven refinery. Southampton, UK: WIT Press. pp. 77–85. ISBN 1-85312-918-6.
- ^ Prasad S, Kannojiya S, Kumar S, Yadav KK, Kundu M, Rakshit A (2021). "Integrative Approaches for Understanding and Designing Strategies of Bioremediation.". In Rakshit A, Parihar M, Sarkar B, Singh HB, Fraceto LF (eds.). Bioremediation Science: From Theory to Practice. CRC Press. ISBN 978-1-000-28046-3.
- ^ Azubuike CC, Chikere CB, Okpokwasili GC (November 2016). "Bioremediation techniques-classification based on site of application: principles, advantages, limitations and prospects". World Journal of Microbiology & Biotechnology. 32 (11): 180. doi:10.1007/s11274-016-2137-x. PMC 5026719. PMID 27638318.
- ^ Kumar V, Shahi SK, Singh S (2018). "Bioremediation: An Eco-sustainable Approach for Restoration of Contaminated Sites". In Singh J, Sharma D, Kumar G, Sharma NR (eds.). Microbial Bioprospecting for Sustainable Development. Singapore: Springer. pp. 115–136. doi:10.1007/978-981-13-0053-0_6. ISBN 978-981-13-0053-0.
- ^ Azubuike CC, Chikere CB, Okpokwasili GC (November 2016). "Bioremediation techniques-classification based on site of application: principles, advantages, limitations and prospects". World Journal of Microbiology & Biotechnology. 32 (11): 180. doi:10.1007/s11274-016-2137-x. PMC 5026719. PMID 27638318.
- ^ Azubuike CC, Chikere CB, Okpokwasili GC (November 2016). "Bioremediation techniques-classification based on site of application: principles, advantages, limitations and prospects". World Journal of Microbiology & Biotechnology. 32 (11): 180. doi:10.1007/s11274-016-2137-x. PMC 5026719. PMID 27638318.
- ^ Ghosh M, Singh SP (July 2005). "A Review on Phytoremediation of Heavy Metals and Utilization of Its Byproducts". Asian Journal on Energy and Environment. 6 (4): 214–231. doi:10.15666/AEER/0301_001018. S2CID 15886743.
- ^ Ford RG, Wilkin RT, Puls RW (2007). Monitored natural attenuation of inorganic contaminants in groundwater, Volume 1 Technical basis for assessment (PDF). U.S. Environmental Protection Agency, EPA/600/R-07/139. OCLC 191800707.
- ^ Ford RG, Wilkin RT, Puls RW (2007). Monitored Natural Attenuation of Inorganic Contaminants in Groundwater, Volume 2 - Assessment for Non-Radionulcides Including Arsenic, Cadmium, Chromium, Copper, Lead, Nickel, Nitrate, Perchlorate, and Selenium (PDF). USEPA.
- ^ Williams KH, Bargar JR, Lloyd JR, Lovley DR (June 2013). "Bioremediation of uranium-contaminated groundwater: a systems approach to subsurface biogeochemistry". Current Opinion in Biotechnology. 24 (3): 489–97. doi:10.1016/j.copbio.2012.10.008. PMID 23159488.
- ^ Ford RG, Wilkin RT, Puls RW (2007). Monitored natural attenuation of inorganic contaminants in groundwater, Volume 3 Assessment for Radionuclides Including Tritium, Radon, Strontium, Technetium, Uranium, Iodine, Radium, Thorium, Cesium, and Plutonium-Americium (PDF). U.S. Environmental Protection Agency, EPA/600/R-10/093.
- ^ a b Palmisano A, Hazen T (2003). Bioremediation of Metals and Radionuclides: What It Is and How It Works (2nd ed.). Lawrence Berkeley National Laboratory. OCLC 316485842.
- ^ Ansari MI, Malik A (November 2007). "Biosorption of nickel and cadmium by metal resistant bacterial isolates from agricultural soil irrigated with industrial wastewater". Bioresource Technology. 98 (16): 3149–53. Bibcode:2007BiTec..98.3149A. doi:10.1016/j.biortech.2006.10.008. PMID 17166714.
- ^ Durán U, Coronado-Apodaca KG, Meza-Escalante ER, Ulloa-Mercado G, Serrano D (May 2018). "Two combined mechanisms responsible to hexavalent chromium removal on active anaerobic granular consortium". Chemosphere. 198: 191–197. Bibcode:2018Chmsp.198..191D. doi:10.1016/j.chemosphere.2018.01.024. PMID 29421729.
- ^ Tripathi M, Munot HP, Shouche Y, Meyer JM, Goel R (May 2005). "Isolation and functional characterization of siderophore-producing lead- and cadmium-resistant Pseudomonas putida KNP9". Current Microbiology. 50 (5): 233–7. doi:10.1007/s00284-004-4459-4. PMID 15886913. S2CID 21061197.
- ^ Yam HM, Leong S, Qiu X, Zaiden N (May 2021). "Bioremediation of Arsenic-Contaminated Water Through Application of Bioengineered Shewanella oneidensis". Irc-Set 2020. Vol. 1. pp. 559–574. doi:10.1007/978-981-15-9472-4_49. ISBN 978-981-15-9471-7. S2CID 236650675.
- ^ Azubuike CC, Chikere CB, Okpokwasili GC (November 2016). "Bioremediation techniques-classification based on site of application: principles, advantages, limitations and prospects". World Journal of Microbiology & Biotechnology. 32 (11): 180. doi:10.1007/s11274-016-2137-x. PMC 5026719. PMID 27638318.
- ^ Fathepure BZ (January 1, 2014). "Recent studies in microbial degradation of petroleum hydrocarbons in hypersaline environments". Frontiers in Microbiology. 5: 173. doi:10.3389/fmicb.2014.00173. PMC 4005966. PMID 24795705.
- ^ Nie J, Sun Y, Zhou Y, Kumar M, Usman M, Li J, et al. (March 2020). "Bioremediation of water containing pesticides by microalgae: Mechanisms, methods, and prospects for future research". The Science of the Total Environment. 707: 136080. Bibcode:2020ScTEn.70736080N. doi:10.1016/j.scitotenv.2019.136080. PMID 31869621.
- ^ Alvarez A, Saez JM, Davila Costa JS, Colin VL, Fuentes MS, Cuozzo SA, et al. (January 2017). "Actinobacteria: Current research and perspectives for bioremediation of pesticides and heavy metals". Chemosphere. 166: 41–62. Bibcode:2017Chmsp.166...41A. doi:10.1016/j.chemosphere.2016.09.070. hdl:11336/63289. PMID 27684437.
- ^ a b Mohapatra D, Rath SK, Mohapatra PK (May 2, 2022). "Soil Fungi for Bioremediation of Pesticide Toxicants: A Perspective". Geomicrobiology Journal. 39 (3–5): 352–372. Bibcode:2022GmbJ...39..352M. doi:10.1080/01490451.2021.2019855. ISSN 0149-0451.
- ^ Nie J, Sun Y, Zhou Y, Kumar M, Usman M, Li J, et al. (March 2020). "Bioremediation of water containing pesticides by microalgae: Mechanisms, methods, and prospects for future research". The Science of the Total Environment. 707: 136080. Bibcode:2020ScTEn.70736080N. doi:10.1016/j.scitotenv.2019.136080. PMID 31869621.
- ^ Chaurasia AK, Adhya TK, Apte SK (December 2013). "Engineering bacteria for bioremediation of persistent organochlorine pesticide lindane (γ-hexachlorocyclohexane)". Bioresource Technology. 149: 439–445. Bibcode:2013BiTec.149..439C. doi:10.1016/j.biortech.2013.09.084. PMID 24135568.
- ^ Alvarez A, Saez JM, Davila Costa JS, Colin VL, Fuentes MS, Cuozzo SA, et al. (January 2017). "Actinobacteria: Current research and perspectives for bioremediation of pesticides and heavy metals". Chemosphere. 166: 41–62. Bibcode:2017Chmsp.166...41A. doi:10.1016/j.chemosphere.2016.09.070. hdl:11336/63289. PMID 27684437.
- ^ a b c d e Juwarkar AA, Singh SK, Mudhoo A (2010). "A comprehensive overview of elements in bioremediation". Reviews in Environmental Science and Bio/Technology. 9 (3): 215–88. Bibcode:2010RESBT...9..215J. doi:10.1007/s11157-010-9215-6. S2CID 85268562.
- ^ a b Boopathy R (2000). "Factors limiting bioremediation technologies". Bioresource Technology. 74 (1): 63–7. Bibcode:2000BiTec..74...63B. doi:10.1016/S0960-8524(99)00144-3. S2CID 1027603.
- ^ a b Wexler P (2014). Encyclopedia of toxicology (3rd ed.). San Diego, Ca: Academic Press Inc. p. 489. ISBN 978-0-12-386454-3.
- ^ Maymó-Gatell X, Chien Y, Gossett JM, Zinder SH (June 1997). "Isolation of a bacterium that reductively dechlorinates tetrachloroethene to ethene". Science. 276 (5318): 1568–71. doi:10.1126/science.276.5318.1568. PMID 9171062.
- ^ a b Sharma J (2019). "Advantages and Limitations of In Situ Methods of Bioremediation". Recent Adv Biol Med. 5 (2019): 10941. doi:10.18639/RABM.2019.955923 (inactive November 1, 2024).
{{cite journal}}: CS1 maint: DOI inactive as of November 2024 (link) - ^ Nie J, Sun Y, Zhou Y, Kumar M, Usman M, Li J, et al. (March 2020). "Bioremediation of water containing pesticides by microalgae: Mechanisms, methods, and prospects for future research". The Science of the Total Environment. 707: 136080. Bibcode:2020ScTEn.70736080N. doi:10.1016/j.scitotenv.2019.136080. PMID 31869621.
- ^ a b Odukkathil G, Vasudevan N (2013). "Toxicity and bioremediation of pesticides in agricultural soil". Reviews in Environmental Science and Bio/Technology. 12 (4): 421–444. Bibcode:2013RESBT..12..421O. doi:10.1007/s11157-013-9320-4. ISSN 1569-1705. S2CID 85173331.
- ^ Supreetha K, Rao SN, Srividya D, Anil HS, Kiran S (August 2019). "Advances in cloning, structural and bioremediation aspects of nitrile hydratases". Molecular Biology Reports. 46 (4): 4661–4673. doi:10.1007/s11033-019-04811-w. PMID 31201677. S2CID 189819253.
- ^ United States Environmental Protection Agency (2012). "A Citizen's Guide to Bioremediation" (PDF). National Service Center for Environmental Publications.
- ^ Lovley DR (October 2003). "Cleaning up with genomics: applying molecular biology to bioremediation". Nature Reviews. Microbiology. 1 (1): 35–44. doi:10.1038/nrmicro731. PMID 15040178. S2CID 40604152.
- ^ Menn FM, Easter JP, Sayler GS (2001). "Genetically Engineered Microorganisms and Bioremediation". Biotechnology Set. pp. 441–63. doi:10.1002/9783527620999.ch21m. ISBN 978-3-527-62099-9.
- ^ Ripp S, Nivens DE, Ahn Y, Werner C, Jarrell J, Easter JP, et al. (2000). "Controlled Field Release of a Bioluminescent Genetically Engineered Microorganism for Bioremediation Process Monitoring and Control". Environmental Science & Technology. 34 (5): 846–53. Bibcode:2000EnST...34..846R. doi:10.1021/es9908319.
- ^ a b Davison J (December 2005). "Risk mitigation of genetically modified bacteria and plants designed for bioremediation". Journal of Industrial Microbiology & Biotechnology. 32 (11–12): 639–50. doi:10.1007/s10295-005-0242-1. PMID 15973534. S2CID 7986980.
- ^ Sayler GS, Ripp S (June 2000). "Field applications of genetically engineered microorganisms for bioremediation processes". Current Opinion in Biotechnology. 11 (3): 286–9. doi:10.1016/S0958-1669(00)00097-5. PMID 10851144.
- ^ Shanker R, Purohit HJ, Khanna P (1998). "Bioremediation for Hazardous Waste Management: The Indian Scenario". In Irvine RL, Sikdar SK (eds.). Bioremediation Technologies: Principles and Practice. pp. 81–96. ISBN 978-1-56676-561-9.
- ^ Bojar D (May 7, 2018). "Building a circular economy with synthetic biology". Phys.org.
- ^ Finny AS (February 8, 2024). "3D bioprinting in bioremediation: a comprehensive review of principles, applications, and future directions". PeerJ. 12: e16897. doi:10.7717/peerj.16897. PMC 10859081. PMID 38344299. S2CID 267586847.
