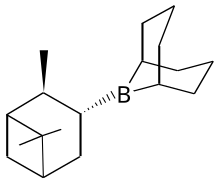
Alpine borane
| |

| |
| Names | |
|---|---|
| IUPAC name
9-(2,6,6-Trimethylbicyclo[3.1.1]hept-3-yl)-9-bora-bicyclo[3.3.1]nonane
| |
| Other names
Alpine-Borane; B-Isopinocampheyl-9-borabicyclo[3.3.1]nonane; B-3-Pinanyl-9-borabicyclo[3.3.1]nonane
| |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.157.575 |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C18H31B | |
| Molar mass | 258.26 g·mol−1 |
| Appearance | Colorless liquid |
| Density | 0.947 g/mL |
| Boiling point | > 55 °C (131 °F; 328 K) |
| Hazards | |
| GHS labelling: | |

| |
| Danger | |
| H250 | |
| P210, P222, P280, P302+P334, P370+P378, P422 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Alpine borane is the commercial name for an organoboron compound that is used in organic synthesis. It is a colorless liquid, although it is usually encountered as a solution. A range of alkyl-substituted borane are specialty reagents in organic synthesis. Two such reagents that are closely related to Alpine borane are 9-BBN and diisopinocampheylborane.
Preparation and reactions
[edit]This reagent is generated by treating 9-BBN with α-pinene.[2]
This sterically crowded chiral trialkylborane can stereoselectively reduce aldehydes in what is known as the Midland Alpine borane reduction, or simply the Midland reduction.[3]
- C8H12B-pinanyl + RCDO → C8H12BOCHDR + (+)-d-pinene
Hydrolysis of the resulting borinic ester affords the alcohol:
- C8H12BOCHDR + H2O → C8H12BOH + HOCHDR
It is also effective for the stereoselective reduction of certain acetylenic ketones.[4] The reaction is proposed to involve formation of an adduct by coordination of the carbonyl oxygen to boron. Intramolecular hydride transfer from the pinane substituent to the carbonyl carbon ensues. Many substrates for the Midland reduction have a low steric group such as an alkyne[5] or a nitrile[6] so as to increase selectivity. Stereochemical control comes from coordination of the carbonyl bulky borane, followed by hydride transfer opposite the largest group.[2][7]

See also
[edit]References
[edit]- ^ R-Alpine-Borane and S-Alpine-Borane at Sigma-Aldrich
- ^ a b M. M. Midland (1989). "Asymmetric reductions with organoborane reagents". Chemical Reviews. 89 (7): 1553–1561. doi:10.1021/cr00097a010.
- ^ M. Mark Midland "B-3-Pinanyl-9-borabicyclo[3.3.1]nonane" in Encyclopedia of Reagents for Organic Synthesis 2001 John Wiley, New York.doi:10.1002/047084289X.rp173. Article Online Posting Date: April 15, 2001
- ^ Midland, M. Mark; Graham, Richard S. (1985). "Asymmetric Reduction of α,β-Acetylenic Ketones with B-3-Pinanyl-9-Borabicyclo[3.3.1]nonane: (R)-(+)-1-Octyln-3-ol". Organic Syntheses. 63: 57. doi:10.15227/orgsyn.063.0057.
- ^ Intramolecular Arene-Alkyne Photocycloaddition M. C. Pirrung J. Org. Chem.; 1987; 52(8); pp 1635 - 1637; doi:10.1021/jo00384a057
- ^ Midland, M. Mark; Lee, Penny E. (1985). "Efficient Asymmetric Reduction of Acyl Cyanides with B-3-Pinanyl 9-BBN (Alpine-Borane)". The Journal of Organic Chemistry. 50 (17): 3237–3239. doi:10.1021/jo00217a053.
- ^ Li, J. J. (2009). Name Reactions, A Collection of Detailed Mechanisms and Synthetic Applications (4th ed.). New York, New York: Springer. pp. 359–360. ISBN 978-3-642-01052-1.

