Ethyl pyruvate
 Ethyl Pyruvate ball and stick model
| |

| |
| Names | |
|---|---|
| Other names
ethyl 2-oxopropanoate
Ethyl-2-oxopropanoat Propanoic acid, 2-oxo-, ethyl ester Pyruvic acid, ethyl ester (8CI) [1] | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.009.557 |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| UN number | 3272 |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C5H8O3 | |
| Molar mass | 116.12 g mol−1 |
| Appearance | colorless liquid |
| Density | 1.045 g cm−3 |
| Melting point | −58 °C (−72 °F; 215 K) |
| Boiling point | 142 °C (288 °F; 415 K) 760 |
| 10 g L−1 (at 20 °C) [2] | |
| log P | 0.048 |
| Hazards[3] | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
Flammable/Irritant |
| GHS labelling: | |

| |
| Warning | |
| H226 | |
| P210, P233, P240, P241, P242, P243, P280, P303+P361+P353, P370+P378, P403+P235, P501 | |
| NFPA 704 (fire diamond) | |
| Flash point | 45 °C |
| Safety data sheet (SDS) | External MSDS |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Ethyl pyruvate is a colorless organic compound with a molecular formula of C5H8O3.
Structure
[edit]Ethyl pyruvate is small molecule with both ketone and ester functionality. The molecule has no hydrogen donors, but three atoms that are hydrogen receptors. Three of the bonds are rotatable and there are no stereocenters.[4] The molecule has two carbonyl carbons, which can act as electrophiles, as well as three α-hydrogens. Ethyl Pyruvate can also be synonymous with ethyl 2-oxopropanoate, Ethyl-2-oxopropanoat, Propanoic acid, 2-oxo-, ethyl ester, Pyruvic acid, and ethyl ester[5]
Research and applications
[edit]Three independent studies of ethyl pyruvate were performed, with rats as their test subjects, and each produced an optimistic result. The first study showed that ethyl pyruvate has a protective role against phosgene-induced pulmonary edema.[6]
The second study showed the therapeutic effects of ethyl pyruvate against severe acute pancreatitis. This study concluded three things: First, ethyl pyruvate prevents the severe acute pancreatitis-induced hepatic expression of tumor necrosis factor α (TNF-α) and interleukin-1β (IL-1β). Second, ethyl pyruvate protects the rats from severe acute pancreatitis-induced liver and pancreas damages. Third, ethyl pyruvate controls liver severe acute pancreatitis induced by NF-κB activation.[7] The third study showed the effects of sodium pyruvate (SP) and ethyl pyruvate (EP) as treatments to brain injury. This experiment concluded that the pyruvate treatments proved beneficial neurologically post-cortical contusion injury (CCI).[8]
The effects of ethyl pyruvate as an antioxidant were compared to that of its sodium salt in a recent study. Ethyl pyruvate has a greater lipophilicity than sodium pyruvate, which allows it to be a more effective scavenger in the reaction. This study was done using a liver homogenate as the model for cell membrane transport deletion.[9] Hypochlorous acid was used as the oxidant, and the focus of the study was on the capacity of the pyruvates to scavenge the reactive oxygen species. Ethyl pyruvate is a good antioxidant due to its α-ketocarboxylate structure, which allows it to reduce hydrogen peroxide to water and scavenge the hydroxyl radical through decarboxylation.
Amino-2H-imidazoles are a new class of BACE-1 inhibitors for the treatment of Alzheimer's disease. Amino-2H-imidazoles were introduced because current treatments of Alzheimer's disease only treat the symptoms, but do not correct the underlying neuropathology. Ethyl pyruvate is used as a reactant in the synthesis of many of these new BACE-1 inhibitors.[10]
Overall, ethyl pyruvate has been found to be beneficial in wound healing, liver disease, pancreatitis, and spinal cord repair. Relating to health, there are still many researchers using ethyl pyruvate in their projects pertaining to myocardial ischemia, reperfusion, and human gastric cancer.[11]
Preparation and reactions
[edit]Ethyl pyruvate can be synthesized in a simple, one-step reaction from the oxidation of ethyl lactate. Since ethyl lactate[12] is slightly cheaper to buy than ethyl pyruvate,[13] this synthesis can be useful. There are many different reagents that can be used to push the reaction forward to yield in excess of 98%, such as using potassium permanganate and aluminum sulfate hydrate in dichloromethane solvent.[14]
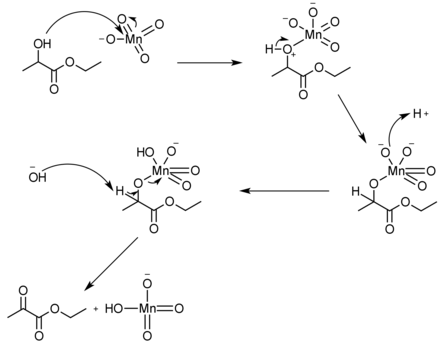
Ethyl pyruvate can undergo reduction (chemistry) as well. For example, when reduced by sodium borohydride the ketone gets reduced to an alcohol, leaving the ester group untouched. But, when ethyl pyruvate is reduced by lithium aluminium hydride, both the ketone and ester get completely reduced to alcohols.
Enantioselective reactions are extremely important in chemistry, as the formation of optically pure products is especially useful in the food, pharmaceutical, and agrochemical industries. An important enantioselective reaction in modern chemistry involves the hydrogenation of α-ketoesters, including ethyl pyruvate.[15] These reactions produce α-hydroxiesters, which are chiral compounds that can be further modified to synthesize important biologically active compounds. In the hydrogenation of ethyl pyruvate, Pt/SiO2 catalysts were modified with a chiral agent, cinchonidine. Without the addition of tin, the enantioselectivity was largely dependent on the size of the particles - larger particles dictated higher enantioselective success. With the promotion of small amounts of tin, the hydrogenation rate and the enantioselective success both increased. However, a critical amount was reached, where additional tin decreased the hydrogenation rate along with the enantioselective success of the reaction.
References
[edit]- ^ "2457 | C5H8O3 | ChemSpider".
- ^ "Ethyl Pyruvate, 98%". Acros Organics. Retrieved 21 March 2013.
- ^ "Ethyl pyruvate natural, 95%". Sigma Aldrich. Retrieved 21 March 2013.
- ^ "Ethyl Pyruvate." ChemExper. N.p., n.d. Web. 13 Mar. 2013. <http://www.chemexper.com/cheminfo/servlet/org.dbcreator.MainServlet?query=entry._entryID=6445>.
- ^ "Chem Spider". Chem Spider. Retrieved 4 October 2013.
- ^ Ethyl pyruvate protects rats from phosgene-induced pulmonary edema by inhibiting cyclooxygenase2 and inducible nitric oxide synthase expression. Chen, Hong-li; Bai, Hua; Xi, Miao-miao; Liu, Riu; Qin, Xu-jun; Liang, Xin; Zhang, Wei; Zhang, Xiao-di; Li, Wen-li; Hai, Chun-xu. Department of Toxicology, Fourth Military Medical University, Xi'an, 710032, China. Epub 2011 Aug 5.
- ^ Therapeutic treatment with ethyl pyruvate attenuates the severity of liver injury in rats with severe acute pancreatitis. Luan, Zheng-Gang; Zhang, Hao; Ma, Xiao-Chun; Zhang, Cheng; Guo, Ren-Xuan. Department of Intensive Care Unit, The First Hospital, China Medical University, Shenyang, China.
- ^ Beneficial effects of sodium or ethyl pyruvate after traumatic brain injury in the rat. Nobuhiro Moro, Richard L. Sutton. Department of Neurosurgery, David Geffen School of Medicine, University of California, Los Angeles, CA, USA. Copyright © 2010 Elsevier Inc.
- ^ Olek, Robert Antonini; Ziolkowski, Wieslaw; Kaczor, Jan Jacek; Wierzba, Tomasz Henryk; Antosiewicz, Jedrzej."Higher Hypochlorous Acid Scavenging Activity of Ethyl Pyruvate Compared to its Sodium Salt" Biosci, Biotechnol, Biochem., 75 (3),(2011). 500-504.
- ^ Gravenfors, Ylva, et al. "New Aminoimidazoles as β-Secretase (BACE-1) Inhibitors Showing Amyloid-β (Aβ) Lowering in Brain." Journal of Medicinal Chemistry 55.21 (2012): 9297-9311.
- ^ "ethyl pyruvate - Compound Summary". Retrieved 11 April 2013.
- ^ "Ethyl lactate". sigmaaldrich.com. Retrieved 15 May 2023.
- ^ "Ethyl pyruvate". sigmaaldrich.com. Retrieved 15 May 2023.
- ^ Preparation of α-keto esters by oxidation of hydroxy esters. Kurata, Takeo; Kobayashi, Makoto; Arimura, Tomohiro; Sekiguchi, Takayuki. Musashino Chemical Laboratory Ltd., Japan. May 9, 2002.
- ^ Ibanez, M.F.; Vetere, V.; Santori, G.F.; Casella, M.L.; Ferretti, O.A."Enantioselective Hydrogenation of Ethyl Pyruvate with Cinchonidine Modified Pt/SiO2 and PtSn/SiO2 Catalysts" The Journal of the Argentine Chemical Society. Vol. 91 (2003). 63-72.

