Iodate
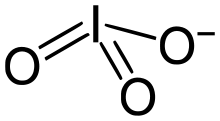 The iodate anion, IO−3
| |
 Space-filling model of the iodate anion
| |
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
| ChEBI |
|
| ChemSpider | |
| 1676 | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| IO3− | |
| Molar mass | 174.902 g·mol−1 |
| Related compounds | |
Related compounds
|
Periodate, Fluoroiodate, Bromate, Chlorate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
An iodate is the polyatomic anion with the formula IO−3. It is the most common form of iodine in nature, as it comprises the major iodine-containing ores.[1] Iodate salts are often colorless. They are the salts of iodic acid.
Structure[edit]
Iodate is pyramidal in structure. The O–I–O angles range from 97° to 105°, somewhat smaller than the O–Cl–O angles in chlorate.[2]
Reactions[edit]
Redox[edit]
Iodate is one of several oxyanions of iodine, and has an oxidation number of +5. It participates in several redox reactions, such as the iodine clock reaction. Iodate shows no tendency to disproportionate to periodate and iodide, in contrast to the situation for chlorate.
Iodate is reduced by sulfite:[1]
- 6HSO−3 + 2IO−3 → 2I− + 6HSO−4
Iodate oxidizes iodide:
- 5I− + IO−3 + 3H2SO4 → 3I2 + 3H2O + 3SO2−4
Similarly, chlorate oxidizes iodide to iodate:
- I− + ClO−3 → Cl− + IO−3
Iodate is also obtained by reducing a periodate with a sulfide. The byproduct of the reaction is a sulfoxide.[3]
Acid-base[edit]
Iodate is unusual in that it forms a strong hydrogen bond with its parent acid:[2]
- IO−3 + HIO3 → H(IO3)−2
The anion H(IO3)−2 is referred to as biiodate.
Principal compounds[edit]
- Calcium iodate, Ca(IO3)2, is the principal ore of iodine. It is also used as a nutritional supplement for cattle.
- Potassium iodate, KIO3, like potassium iodide, has been issued as a prophylaxis against radioiodine absorption in some countries.[4][5] It is also one of the iodine compounds used to make iodized salt.[6]
- Potassium hydrogen iodate (or potassium biiodate), KH(IO3)2, is a double salt of potassium iodate and iodic acid, as well as an acid itself.
- When some oxygen is replaced by fluorine, fluoroiodates are produced.
Natural occurrence[edit]
Minerals containing iodate are found in the caliche deposits of Chile. The most important iodate minerals are lautarite and brüggenite, but also copper-bearing iodates such as salesite are known.[7]
Natural waters contain iodine in the form of iodide and iodate, their ratio being dependent on redox conditions and pH. Iodate is the second most abundant form in water. It is mostly associated with alkaline waters and oxidizing conditions.[8]
References[edit]
- ^ a b Lyday, Phyllis A. (2005). "Iodine and Iodine Compounds". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. pp. 382–390. doi:10.1002/14356007.a14_381. ISBN 978-3527306732.
- ^ a b Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- ^ Qiu, Chao; Sheng Han; Xingguo Cheng; Tianhui Ren (2005). "Distribution of Thioethers in Hydrotreated Transformer Base Oil by Oxidation and ICP-AES Analysis" (abstract). Industrial & Engineering Chemistry Research. 44 (11): 4151–4155. doi:10.1021/ie048833b. Retrieved 2007-05-03.
Thioethers can be oxidized to sulfoxides by periodate, and periodate is reduced to iodate
- ^ "Radiological Protection Institute of Ireland | | Media | Press releases | Radioactivity released from Wylfa nuclear power plant is extremely low and of no health significance". Archived from the original on 2013-10-17. Retrieved 2013-04-08.
- ^ "Decision to Discontinue the Future Distribution of Iodine Tablets". Archived from the original on 2013-10-18. Retrieved 2013-05-22.
- ^ Arroyave, Guillermo; Pineda, Oscar; Scrimshaw, Nevin S. (1956) [May 1955]. "The stability of potassium iodate in crude table salt". Bulletin of the World Health Organization. 14 (1): 183–185. PMC 2538103. PMID 13329845.
- ^ "Home". mindat.org.
- ^ Sweden (13 December 2013). "Iodine (including PVP-iodine) Product types 1, 3, 4, 22 (EU 528/2012 assessment)". pp. 29–30.
