Lanosterol

| |

| |
| Names | |
|---|---|
| IUPAC name
Lanosta-8,24-dien-3β-ol
| |
| Systematic IUPAC name
(1R,3aR,5aR,7S,9aS,11aR)-3a,6,6,9a,11a-Pentamethyl-1-[(2R)-6-methylhept-5-en-2-yl]-2,3,3a,4,5,5a,6,7,8,9,9a,10,11,11a-tetradecahydro-1H-cyclopenta[a]phenanthren-7-ol | |
| Identifiers | |
3D model (JSmol)
|
|
| 2226449 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.001.105 |
| EC Number |
|
| KEGG | |
| MeSH | Lanosterol |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C30H50O | |
| Molar mass | 426.71 g/mol |
| Melting point | 138 to 140 °C (280 to 284 °F; 411 to 413 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Lanosterol is a tetracyclic triterpenoid and is the compound from which all animal and fungal steroids are derived. By contrast, plant steroids are produced via cycloartenol.[1]
Role in the biosynthesis of other steroids[edit]
Elaboration of lanosterol under enzyme catalysis leads to the core structure of steroids. 14-Demethylation of lanosterol by CYP51 eventually yields cholesterol.[2]

Biosynthesis[edit]
| Description | Illustration | Enzyme |
| Two molecules of farnesyl pyrophosphate condense with reduction by NADPH to form squalene | squalene synthase | |
| Squalene is oxidized to 2,3-oxidosqualene (squalene epoxide) | 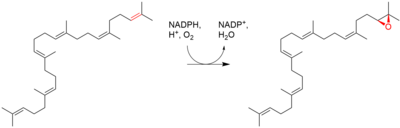 |
squalene monooxygenase |
| 2,3-Oxidosqualene is converted to a protosterol cation and finally to lanosterol |  |
lanosterol synthase |
| (step 2) | 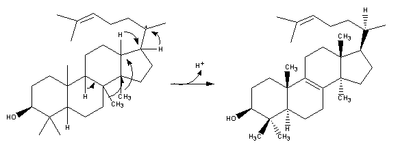 |
(step 2) |
Research[edit]
Lanosterol has been identified as a key component in maintaining eye lens clarity.[3] Pre-clinical research has identified Lanosterol as a possible agent for the reversal and prevention of cataracts.[4] In vivo experiments on dogs showed significant reversal of cataracts within 6 weeks of lanosterol injection.[5] In 2018, Lanosterol was shown to improve lens clarity in cells with lens clouding due to aging or physical stressors.[6] A subsequent study in 2022 by Kehao Wang, Hoshino, Kentaro Uesugi, Naoto Yagi, Pierscionek and Andley found positive results on the optics of the lens in mice with cataracts.[7]
Use[edit]
Lanosterol is an ingredient in over-the-counter ophthalmic products to prevent cataracts. However, the solubility and bioavailability of lanosterol is not conducive to aqueous formulations.[8] Heliostatix Biotechnology claims to have a method of solubilizing lanosterol for use in aqueous products.[9]
See also[edit]
References[edit]
- ^ Schaller, Hubert (May 2003). "The role of sterols in plant growth and development". Progress in Lipid Research. 42 (3): 163–175. doi:10.1016/S0163-7827(02)00047-4. PMID 12689617.
- ^ "Do Lanosterol eye drops work for dogs? - PetACS Pet Health Products". petacs.com. Retrieved 2023-06-06.
- ^ Huff, M; Telford, D (July 2005). "Lord of the rings – the mechanism for oxidosqualene:lanosterol cyclase becomes crystal clear". Trends in Pharmacological Sciences. 26 (7): 335–340. doi:10.1016/j.tips.2005.05.004. PMID 15951028.
- ^ Zhang, K.; Zhao, L.; Zhu, J.; Hou, R.; Wang, S.; Yan, Y. (2016). "Lanosterol reversal of protein aggregation in cataract". Acta Ophthalmologica. 94. doi:10.1111/j.1755-3768.2016.0033.
- ^ Zhao, Ling; Chen, Xiang-Jun; Zhu, Jie; Xi, Yi-Bo; Yang, Xu; Hu, Li-Dan; Ouyang, Hong; Patel, Sherrina H.; Jin, Xin; Lin, Danni; Wu, Frances; Flagg, Ken; Cai, Huimin; Li, Gen; Cao, Guiqun; Lin, Ying; Chen, Daniel; Wen, Cindy; Chung, Christopher; Wang, Yandong; Qiu, Austin; Yeh, Emily; Wang, Wenqiu; Hu, Xun; Grob, Seanna; Abagyan, Ruben; Su, Zhiguang; Tjondro, Harry Christianto; Zhao, Xi-Juan; Luo, Hongrong; Hou, Rui; Jefferson, J.; Perry, P.; Gao, Weiwei; Kozak, Igor; Granet, David; Li, Yingrui; Sun, Xiaodong; Wang, Jun; Zhang, Liangfang; Liu, Yizhi; Yan, Yong-Bin; Zhang, Kang (July 2015). "Lanosterol reverses protein aggregation in cataracts". Nature. 523 (7562): 607–611. Bibcode:2015Natur.523..607Z. doi:10.1038/nature14650. PMID 26200341. S2CID 4469138.
- ^ Shen, Xinyue; Zhu, Manhui; Kang, Lihua; Tu, Yuanyuan; Li, Lele; Zhang, Rutan; Qin, Bai; Yang, Mei; Guan, Huaijin (11 July 2018). "Lanosterol Synthase Pathway Alleviates Lens Opacity in Age-Related Cortical Cataract". Journal of Ophthalmology. 2018: 1–9. doi:10.1155/2018/4125893. PMC 6079410. PMID 30116630.
- ^ Wang, Kehao; Hoshino, Masato; Uesugi, Kentaro; Yagi, Naoto; Pierscionek, Barbara K.; Andley, Usha P. (2022-05-16). "Oxysterol Compounds in Mouse Mutant αA- and αB-Crystallin Lenses Can Improve the Optical Properties of the Lens". Investigative Ophthalmology & Visual Science. 63 (5): 15. doi:10.1167/iovs.63.5.15. ISSN 1552-5783. PMC 9123516. PMID 35575904.
- ^ Daszynski, Damian M.; Santhoshkumar, Puttur; Phadte, Ashutosh S.; Sharma, K. Krishna; Zhong, Haizhen A.; Lou, Marjorie F.; Kador, Peter F. (2019). "Failure of Oxysterols Such as Lanosterol to Restore Lens Clarity from Cataracts". Scientific Reports. 9 (1): 8459. Bibcode:2019NatSR...9.8459D. doi:10.1038/s41598-019-44676-4. PMC 6560215. PMID 31186457.
- ^ Heliostatix.org
Further reading[edit]
- Corey, E. J.; Russey, William E.; de Montellano, Paul R. Ortiz (October 1966). "2,3-Oxidosqualene, an Intermediate in the Biological Synthesis of Sterols from Squalene". Journal of the American Chemical Society. 88 (20): 4750–4751. doi:10.1021/ja00972a056. PMID 5918046.
- Abe, Ikuro.; Rohmer, Michel.; Prestwich, Glenn D. (1 September 1993). "Enzymatic cyclization of squalene and oxidosqualene to sterols and triterpenes". Chemical Reviews. 93 (6): 2189–2206. doi:10.1021/cr00022a009.
- Eschenmoser, A.; Ruzicka, L.; Jeger, O.; Arigoni, D. (1955). "Zur Kenntnis der Triterpene. 190. Mitteilung. Eine stereochemische Interpretation der biogenetischen Isoprenregel bei den Triterpenen" [For knowledge of the triterpenes. Part 190. A stereochemical interpretation of the biogenetic isoprene rule in triterpenes]. Helvetica Chimica Acta (in German). 38 (7): 1890–1904. doi:10.1002/hlca.19550380728.
- Wang, Kehao; Hoshino, Masato; Uesugi, Kentaro; Yagi, Naoto; Pierscionek, Barbara K.; Andley, Usha P. (2022-05-16). "Oxysterol Compounds in Mouse Mutant αA- and αB-Crystallin Lenses Can Improve the Optical Properties of the Lens". Investigative Ophthalmology & Visual Science. 63 (5): 15. doi:10.1167/iovs.63.5.15. ISSN 1552-5783. PMC 9123516. PMID 35575904.
