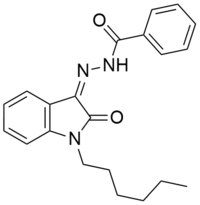
MDA-19
 | |
| Legal status | |
|---|---|
| Legal status |
|
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C21H23N3O2 |
| Molar mass | 349.434 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
MDA-19 (also known as BZO-HEXOXIZID)[2][3] is a drug that acts as a potent and selective agonist for the cannabinoid receptor CB2, with reasonable selectivity over the psychoactive CB1 receptor, though with some variation between species. In animal studies it was effective for the treatment of neuropathic pain, but did not affect rat locomotor activity in that specific study. The pharmacology of MDA-19 in rat cannabinoid receptors have been demonstrated to function differently than human cannabinoid receptors with MDA-19 binding to human CB1 receptors 6.9× higher than rat CB1 receptors.[4][5]
Discovery
[edit]MDA-19 was first synthesized and studied in the late 2000s by researchers at the University of Texas MD Anderson Cancer Center.[6] [7][5]
Pharmacology
[edit]MDA-19 binds to human CB2 receptors at Ki = 43.3 ± 10.3 nM and human CB1 receptors at Ki = 162.4 ± 7.6 nM and functions as an agonist in human cannabinoid receptors but functions differently in rat cannabinoid receptors binding to rat cannabinoid CB2 receptors at Ki = 16.3 ± 2.1 and CB1 receptors at Ki = 1130 ± 574 nM binding to rat CB1 receptors 6.9× weaker than human CB1 receptors but increased binding for CB2. MDA-19 is an agonist at human CB1 and CB2 receptors as well as rat CB1 receptors but functions as an inverse agonist in rat CB2 receptors.[4]
Society and Culture
[edit]MDA-19 along with its shortened Pentyl tailchain analog (MDA-19-Pentyl / 5Carbon-MDA-19/ BZO-POXIZID)[8] and its 5-Fluoro Pentyl analog (5F-MDA-19 /5F-BZO-POXIZID)[9] and its Cyclohexylmethyl analog (CHM-MDA-19 / BZO-CHMOXIZID)[7] was identified in synthetic smoke blends seized in the United States as early as September 2021.[8][7][9][10] United States Border Protection Officers identified BZO-4en-POXIZID (also known as 4en-pentyl-MDA-19) as early as February, 2022[11] The Center for Forensic Science Research & Education (CFSRE) analyzed 11 samples of suspected synthetic smoke blends between May and September 2022 within the Philadelphia area and found the pentyl analog of MDA-19 in 5 out of 11 samples.[12] Despite their reported lower CB1 binding affinity, other low CB1 binding synthetic cannabinoids such as UR-144 (Ki = 150 nM CB1 and Ki = 1.8 nM CB2) and XLR-11 (EC50 values of 98 nM CB1 and 83 nM CB2) have been previously identified in smoke blends in 2012.[13][14][15][16]
Legality
[edit]In the United States, As of October 20, 2024 MDA-19 is legal at the federal level, but may be considered illegal if intended for human consumption under the federal analogue act.[17]
North Dakota has placed MDA-19 (along with BZO-CHMOXIZID (CHM-MDA-19), BZO-POXIZID (Pentyl MDA-19), 5F-BZO-POXIZID (5F-MDA-19) and BZO-4en-POXIZID (4en-pentyl MDA-19) into Schedule I on 04/27/23.[18]
In China, the May 2021 ban on specific synthetic cannabinoid core classes does not include the class of cannabinoids MDA-19 belongs to.[19][20]
See also
[edit]References
[edit]- ^ Anvisa (2023-07-24). "RDC Nº 804 - Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial" [Collegiate Board Resolution No. 804 - Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control] (in Brazilian Portuguese). Diário Oficial da União (published 2023-07-25). Archived from the original on 2023-08-27. Retrieved 2023-08-27.
- ^ "Systematic Naming for MDA 19".
- ^ "New Systematic Naming for Synthetic Cannabinoid "MDA-19" and its Related Analogues: BZO-HEXOXIZID, 5F-BZO-POXIZID, and BZO-POXIZID". 31 August 2021.
- ^ a b Xu JJ, Diaz P, Astruc-Diaz F, Craig S, Munoz E, Naguib M (July 2010). "Pharmacological Characterization of a Novel Cannabinoid Ligand, MDA19, for Treatment of Neuropathic Pain". Anesthesia and Analgesia. 111 (1): 99–109. doi:10.1213/ANE.0b013e3181e0cdaf. PMC 3253719. PMID 20522703.
- ^ a b Diaz P, Xu J, Astruc-Diaz F, Pan HM, Brown DL, Naguib M (August 2008). "Design and Synthesis of a Novel Series of N-Alkyl Isatin Acylhydrazone Derivatives that Act as Selective Cannabinoid Receptor 2 Agonists for the Treatment of Neuropathic Pain". Journal of Medicinal Chemistry. 51 (16): 4932–4947. doi:10.1021/jm8002203. PMID 18666769.
- ^ US 20180200225, Attala MN, Diaz P, "Hydrazone modulators of cannabinoid receptors", published 19 July 2018, assigned to Cleveland Clinic Foundation
- ^ a b c "BZO-CHMOXIZID" (PDF).
- ^ a b "BZO-POXIZID" (PDF).
- ^ a b "5F-BZO-POXIZID" (PDF).
- ^ "BZO-HEXOXIZID" (PDF).
- ^ "CBP Officers and Scientists Identify Two New Synthetic Cannabinoid Analogues | U.S. Customs and Border Protection Preview".
- ^ Krotulski AJ, Shinefeld J, DeBord J, Teixeira da Silva D, Logan BK (September 2022). "Quarterly Drug Checking Report" (PDF). Department of Health, City of Philadelphia. p. 2. Archived from the original (PDF) on 2022-12-16.
- ^ United States v. Earl Ramos (Court of Appeals for the Eighth Circuit 9 February 2016), Text.
- ^ Techau KW. "Synthetic Drug Sales Send a Mother and Her Son to Federal Prison" (PDF). United States Attorney’s Office Northern District of Iowa.
- ^ Frost JM, Dart MJ, Tietje KR, Garrison TR, Grayson GK, Daza AV, et al. (January 2010). "Indol-3-ylcycloalkyl ketones: effects of N1 substituted indole side chain variations on CB(2) cannabinoid receptor activity". Journal of Medicinal Chemistry. 53 (1): 295–315. doi:10.1021/jm901214q. PMID 19921781.
- ^ Banister SD, Stuart J, Kevin RC, Edington A, Longworth M, Wilkinson SM, et al. (August 2015). "Effects of Bioisosteric Fluorine in Synthetic Cannabinoid Designer Drugs JWH-018, AM-2201, UR-144, XLR-11, PB-22, 5F-PB-22, APICA, and STS-135". ACS Chemical Neuroscience. 6 (8): 1445–1458. doi:10.1021/acschemneuro.5b00107. PMID 25921407.
- ^ "21 U.S. Code § 813 - Treatment of controlled substance analogues".
- ^ "AN ACT to amend and reenact sections 19-03.1-05, 19-03.1-11, and 19-03.1-13 of the North Dakota Century Code, relating to the scheduling of controlled substances; and to declare an emergency" (PDF). Sixty-eighth Legislative Assembly of North Dakota in Regular Session. 3 January 2023.
- ^ "关于将合成大麻素类物质和氟胺酮等18种物质列入《非药用类麻醉药品和精神药品管制品种增补目录》的公告" [Announcement on the inclusion of 18 substances including synthetic cannabinoids and fluamine in the "Additional Catalogue of Controlled Varieties of Non-medicinal Narcotics and Psychotropic Drugs"]. Ministry of Public Security of the People's Republic of China (in Chinese). 12 May 2021.
- ^ "Details for Country CHINA".
