Chlormethine
| |

| |
| Names | |
|---|---|
| IUPAC name
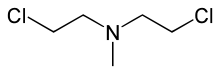
2-Chloro-N-(2-chloroethyl)-N-methylethanamine
| |
| Other names | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.000.110 |
| EC Number |
|
| KEGG | |
| MeSH | Mechlorethamine |
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| UN number | 2810 |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C5H11Cl2N | |
| Molar mass | 156.05 g·mol−1 |
| Appearance | Colorless liquid |
| Odor | Fishy, ammoniacal |
| log P | 0.91 |
| Pharmacology | |
| D08AX04 (WHO) L01AA05 (WHO) | |
| |
| |
| Pharmacokinetics: | |
| <1 minute | |
| 50% (Kidney) | |
| Legal status | |
| Related compounds | |
Related amines
|
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Chlormethine (INN, BAN), also known as mechlorethamine (USAN, USP), mustine, HN2, and (in post-Soviet states) embikhin (эмбихин), is a nitrogen mustard sold under the brand name Mustargen among others. It is the prototype of alkylating agents, a group of anticancer chemotherapeutic drugs. It works by binding to DNA, crosslinking two strands and preventing cell duplication. It binds to the N7 nitrogen on the DNA base guanine. As the chemical is a blister agent, its use is strongly restricted within the Chemical Weapons Convention where it is classified as a Schedule 1 substance.
Mechlorethamine belongs to the group of nitrogen mustard alkylating agents.[4][5][6]
Uses
[edit]It has been derivatized into the estrogen analogue estramustine phosphate, used to treat prostate cancer. It can also be used in chemical warfare where it has the code-name HN2. This chemical is a form of nitrogen mustard gas and a powerful vesicant. Historically, some uses of mechlorethamine have included lymphoid malignancies such as Hodgkin's disease, lymphosarcoma, chronic myelocytic leukemia, polycythemia vera, and bronchogenic carcinoma [7] Mechlorethamine is often administered intravenously,[8] but when compounded into a topical formulation it can also be used to treat skin diseases. There have been studies demonstrating that topical administration of mechlorethamine has efficacy in mycosis fungoides-type cutaneous T cell lymphoma.[9][10][11]
Another use of chlormethine is in the synthesis of pethidine (meperidine).[12]
Side effects and toxicity
[edit]Mechlorethamine is a highly toxic medication, especially for women who are pregnant, breastfeeding, or of childbearing age.[13][14] At high enough levels, exposure can be fatal.[6]
The adverse effects of mechlorethamine depend on the formulation.[15] When used in chemical warfare, it can cause immunosuppression and damage to mucous membranes of the eyes, skin, and respiratory tract. Mucous membranes and damp or damaged skin are more affected by exposure to HN-2. Though symptoms of exposure are generally delayed, the DNA damage it causes occurs very quickly. More serious exposures cause symptoms to develop sooner. Eye symptoms develop first, in the first 1–2 hours (severe exposure) or 3–12 hours (mild to moderate exposure) followed by airway (2-6/12–24 hours) and skin symptoms (6–48 hours). Hot, humid weather shortens the latent (symptom-free) period.[6]
Symptoms of toxic exposure to HN-2 vary based on the route of exposure. Eye exposure causes lacrimation (tear production), burning, irritation, itching, a feeling of grittiness or dryness, blepharospasm (spasms of the eyelid), and miosis (pinpoint pupils). More severe cases cause edema (swelling from fluid accumulation) in the eyelids, photophobia (extreme sensitivity to light), severe pain, corneal ulceration, and blindness.[6]
Inhalation of chlormethine damages the upper and lower airways sequentially, with more severe exposures causing faster damage that afflicts lower parts of the respiratory tract. Early symptoms include rhinorrhea (runny nose), epistaxis (nosebleed), toneless voice, sneezing, barking cough, and dyspnea (in smokers and asthmatics). Later symptoms include pain in the nose/sinuses and inflammation of the airway. In severe cases, there may be epithelial necrosis throughout the respiratory tract, causing pseudomembrane formation, which can obstruct the airway. Pneumonia may develop and prove fatal.[6]
Skin exposure mainly causes erythema (redness) and vesication (blistering) at first, but absorption through the skin causes systemic toxicity. In cases where more than 25% of the skin is affected, fatal exposure is likely to have occurred.[6]
Though ingestion is uncommon, if mechlorethamine is swallowed it causes severe chemical burns to the gastrointestinal tract and concomitant nausea, vomiting, diarrhea, abdominal pain, and hemorrhage.[6]
Long-term effects of acute or chronic chlormethine exposure are caused by damage to the immune system. White blood cell counts drop, increasing the risk of infection, and red blood cell and platelet counts may also drop due to bone marrow damage. Chronic eye infections may result from exposure, but blindness is temporary. Long-term effects on the respiratory system include anosmia (inability to smell), ageusia (inability to taste), inflammation, chronic infections, fibrosis, and cancer. Skin that has been damaged by HN2 can change pigmentation or become scarred, and may eventually develop cancer.[6]
History
[edit]The effect of vesicant (blister) agents in the form of mustard gas (sulfur mustard, Bis(2-chloroethyl) sulfide) on bone marrow and white blood cells had been known since the First World War.[16] In 1935 several lines of chemical and biological research yielded results that would be explored after the start of the Second World War. The vesicant action of a family of chemicals related to the sulfur mustards, but with nitrogen substituting for sulfur was discovered—the "nitrogen mustards" were born.[17] The particular nitrogen mustard chlormethine (mechlorethamine) was first synthesized.[18] And the action of sulfur mustard on tumors in laboratory animals was investigated for the first time.[19]
After the US entry into the Second World War the nitrogen mustards were candidate chemical warfare agents and research on them was initiated by the Office of Scientific Research and Development (OSRD). The OSRD let contracts to study them to two universities—Yale University and the University of Chicago. Inspired perhaps by the preliminary research in 1935, independently both groups thought to test whether a medically useful differential toxicity between animals and animal tumors existed.[20] The Yale pharmacologists Louis Goodman and Alfred Gilman were the first to conduct a clinical trial, on 27 August 1942, using the agent HN3 (tris(2-chloroethyl)amine) on a patient known as J.D.[21][22][23]
The next year the Chicago group, led by Leon O. Jacobson, conducted trials with HN2 (chlormethine) which was the only agent in this group to see eventual clinical use. Wartime secrecy prevented any of this ground-breaking work on chemotherapy from being published, but papers were released once wartime secrecy ended, in 1946.[24]
Chemistry
[edit]Chlormethine is combustible and becomes explosive under extreme conditions. It can react with metals to form gaseous hydrogen.[6]
References
[edit]- ^ a b c "Ledaga". Therapeutic Goods Administration (TGA). 30 June 2021. Archived from the original on 5 September 2021. Retrieved 5 September 2021.
- ^ a b "Ledaga EPAR". European Medicines Agency. 17 September 2018. Archived from the original on 5 September 2021. Retrieved 5 September 2021.
- ^ "Health product highlights 2021: Annexes of products approved in 2021". Health Canada. 3 August 2022. Retrieved 25 March 2024.
- ^ Rappeneau S, Baeza-Squiban A, Jeulin C, Marano F (March 2000). "Protection from cytotoxic effects induced by the nitrogen mustard mechlorethamine on human bronchial epithelial cells in vitro". Toxicol. Sci. 54 (1): 212–21. doi:10.1093/toxsci/54.1.212. PMID 10746948.
- ^ "Chapter 3: Principles of Oncologic Pharmacotherapy". Cancer Management: A Multidisciplinary Approach. 2010. Archived from the original on 15 May 2009. Retrieved 8 October 2023.
- ^ a b c d e f g h i "CDC - The Emergency Response Safety and Health Database: Blister Agent: NITROGEN MUSTARD HN-2 - NIOSH". U.S. Centers for Disease Control and Prevention (CDC). Archived from the original on 2019-06-28. Retrieved 2016-04-20.
- ^ Bunn Jr, P. A.; Hoffman, S. J.; Norris, D; Golitz, L. E.; Aeling, J. L. (1994). "Systemic therapy of cutaneous T-cell lymphomas (mycosis fungoides and the Sézary syndrome)". Annals of Internal Medicine. 121 (8): 592–602. doi:10.7326/0003-4819-121-8-199410150-00007. PMID 8085692. S2CID 22909007.
- ^ Medline (2012). Mechlorethamine. Retrieved from https://www.nlm.nih.gov/medlineplus/druginfo/meds/a682223.html Archived 2016-07-05 at the Wayback Machine
- ^ Lindahl LM, Fenger-Gron M, Iversen L. Topical nitrogen mustard therapy in patients with mycosis fungoides or parapsoriasis. J Eur Acad Dermatol Venereol. 2013 Feb;27(2):163-8.
- ^ Galper SL, Smith BD, Wilson LD. Diagnosis and management of mycosis fungoides. Oncology (Williston Park). 2010 May;24(6):491-501.
- ^ Lessin SR, Duvic M, Guitart J, Pandya AG, Strober BE, Olsen EA, Hull CM, Knobler EH, Rook AH, Kim EJ, Naylor MF, Adelson DM, Kimball AB, Wood GS, Sundram U, Wu H, Kim YH. Topical chemotherapy in cutaneous T-cell lymphoma: positive results of a randomized, controlled, multicenter trial testing the efficacy and safety of a novel mechlorethamine, 0.02%, gel in mycosis fungoides. JAMA Dermatol. 2013 Jan;149(1):25-32.
- ^ McErlane, KM; Wood, RJ; Matsui, F; Lovering, EG (July 1978). "Impurities in Drugs II: Meperidine and Its Formulations". Journal of Pharmaceutical Sciences. 67 (7): 958–961. doi:10.1002/jps.2600670723. PMID 660515.
- ^ Recordati Rare Diseases Inc. (2013). Mustargen Package Insert. Retrieved from https://www.drugs.com/pro/mustargen.html Archived 2018-09-20 at the Wayback Machine
- ^ Actelion Pharmaceuticals Ltd. (2013) Valchlor Package Insert. Retrieved from http://www.accessdata.fda.gov/drugsatfda_docs/label/2013/202317lbl.pdf Archived 2021-03-28 at the Wayback Machine
- ^ Mustargen Archived 2018-09-20 at the Wayback Machine and Valchlor Archived 2021-03-28 at the Wayback Machine
- ^ Krumbhaar EB, Krumbhaar HD (1919). "The blood and bone marrow in yellow cross gas (mustard gas) poisoning: changes produced in the bone marrow of fatal cases". J Med Res. 40 (5): 497–508. doi:10.1016/0002-9610(63)90232-0. PMID 13947966. Archived from the original on 2020-02-10. Retrieved 2018-10-14.
- ^ Ward, Kyle (1935). "The Chlorinated Ethylamines — A New Type of Vesicant". J. Am. Chem. Soc. 57 (5): 914–916. doi:10.1021/ja01308a041.
- ^ V. Prelog, V. Štěpán (1935). "Nouvele synthèse des pipérazines N-monoalcoylées (VIIième communication sur les bis-[β-halogénoéthyl]-amines)". Collection of Czechoslovak Chemical Communications. 7: 93–102. doi:10.1135/cccc19350093.
- ^ Berenblum I. (1935). "Experimental inhibition of tumor induction by mustard gas and other compounds". Journal of Pathology and Bacteriology. 40 (3): 549–558. doi:10.1002/path.1700400312.
- ^ Einhorn, J. (1985). "Nitrogen mustard: the origin of chemotherapy for cancer". Int J Radiat Oncol Biol Phys. 11 (7): 1375–1378. doi:10.1016/0360-3016(85)90254-8. PMID 3891698.
- ^ Alfred Gilman, Frederick S. Philips (1946). "The Biological Actions and Therapeutic Applications of the B-Chloroethyl Amines and Sulfides". Science. 103 (2675): 409–436. Bibcode:1946Sci...103..409G. doi:10.1126/science.103.2675.409. PMID 17751251.
- ^ Gilman, Alfred (1963). "The initial clinical trial of nitrogen mustard". Am J Surg. 105 (5): 574–578. doi:10.1016/0002-9610(63)90232-0. PMID 13947966.
- ^ Fenn, John E.; et al. (2011). "First Use of Intravenous Chemotherapy Cancer Treatment: Rectifying the Record". Journal of the American College of Surgeons. 212 (3): 413–417. doi:10.1016/j.jamcollsurg.2010.10.018. PMID 21247779.
- ^ Jacobson L.O., Spurr C.L., Barron E., Smith T., Lushbaugh C., Dick G.F. (1946). "Nitrogen Mustard Therapy: Studies on the Effect of Methyl-Bis (Beta-Chloroethyl) Amine Hydrochloride on Neoplastic Diseases and Allied Disorders of the Hemopoietic System". JAMA. 132 (2675): 263–271. doi:10.1001/jama.1946.02870400011003. PMID 20997209.
{{cite journal}}: CS1 maint: multiple names: authors list (link)
