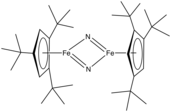
Metal nitrido complex
Metal nitrido complexes are coordination compounds and metal clusters that contain an atom of nitrogen bound only to transition metals. These compounds are molecular, i.e. discrete in contrast to the polymeric, dense nitride materials that are useful in materials science.[1] The distinction between the molecular and solid-state polymers is not always very clear as illustrated by the materials Li6MoN4 and more condensed derivatives such as Na3MoN3. Transition metal nitrido complexes have attracted interest in part because it is assumed that nitrogen fixation proceeds via nitrido intermediates. Nitrido complexes have long been known, the first example being salts of [OsO3N]−, described in the 19th century.[2]
Structural trends
[edit]Mononuclear complexes feature terminal nitride ligands, typically with short M-N distances consistent with metal ligand multiple bonds. For example, in the anion in PPh4[MoNCl4], the Mo-N distance is 163.7 pm. The occurrence of terminal nitrido ligands follow the patterns seen for oxo complexes: they are more common for early and heavier metals. Many bi- and polynuclear complexes are known with bridging nitrido ligands.[3] More exotic metal nitrido complexes are also possible, such as a recently reported compound containing a terminal uranium nitride (-U≡N) bond.[4]
- Example metal nitrido complexes
-
[OsNO3]−, isoelectronic with osmium tetroxide.
-
[MoNCl4]−, a square pyramidal Mo(VI) complex.
-
[W2(μ-N)Cl10]−, containing two W(VI) centres bridged by a nitrido ligand.
-
[Ir3N(SO4)6(H2O)3]4−, structurally related to basic iron acetate.
-
A uranium nitrido complex.
Preparative routes
[edit]Metal nitrides are produced using a variety of nitrogen sources. The first example above is prepared from amide (NH2−) as the N3− source:[5]
- OsO4 + KNH2 → KOsO3N + H2O
Most commonly however, nitrido complexes are produced by decomposition of azido complexes.[6] The driving force for these reactions is the great stability of N2. Nitrogen trichloride is an effective reagent to give chloro-nitrido complexes. In some cases, even N2 and nitriles can serve as sources of nitride ligands.[7]
Reactions of nitrido ligands
[edit]The nitride ligand can be both electrophilic and nucleophilic.[8][9] Terminal nitrides of early metals tend to be basic and oxidizable, whereas nitrides of the later metals tend to be oxidizing and electrophilic. The former behavior is illustrated by their N-protonation and N-alkylation. Ru and Os nitrido complexes often add organophosphines to give iminophosphine derivatives containing the R3PN− ligand.
Interstitial nitrides
[edit]Owing to the ability of nitrido ligands to serve as a bridging ligand, several metal clusters are known to contain nitride ligands at their center. Such nitrido ligands are termed interstitial. In some cases, the nitride is completely encased in the center of six or more metals and cannot undergo reactions, although it contributes to the intermetallic bonding.[10]

See also
[edit]General references
[edit]- ^ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- ^ Fritzsche, J.; Struve, H. (1847). "Ueber die Osman-Osmiumsäure". Journal für Praktische Chemie. 41 (1): 97–113. doi:10.1002/prac.18470410113.
- ^ Dehnicke, Kurt; Strähle, Joachim (1992). "Nitrido Complexes of Transition Metals". Angewandte Chemie International Edition in English. 31 (8): 955–978. doi:10.1002/anie.199209551.
- ^ King, David M.; Tuna, Floriana; McInnes, Eric J. L.; McMaster, Jonathan; Lewis, William; Blake, Alexander J.; Liddle, Stephen T. (2012). "Synthesis and Structure of a Terminal Uranium Nitride Complex". Science. 337 (6095): 717–720. Bibcode:2012Sci...337..717K. doi:10.1126/science.1223488. PMID 22745250.
- ^ Grube, H. L. (1956). "The platinum metals: Potassium osmiamate". In Brauer, Georg (ed.). Handbook of Preparative Inorganic Chemistry. Vol. 2. Translated by Stecher, Paul G. (2nd ed.). NY, NY: Academic Press. p. 1605. LCCN 63-14307 – via the Internet Archive.
- ^ Reiners, Matthias; Maekawa, Miyuki; Daniliuc, Constantin G.; Freytag, Matthias; Jones, Peter G.; White, Peter S.; Hohenberger, Johannes; Sutter, Jörg; Meyer, Karsten; Maron, Laurent; Walter, Marc D. (2017). "Reactivity studies on [Cp′Fe(μ-I)]2: Nitrido-, sulfido- and diselenide iron complexes derived from pseudohalide activation". Chemical Science. 8 (5): 4108–4122. doi:10.1039/C7SC00570A. PMC 6099922. PMID 30155215.
- ^ Laplaza, Catalina E.; Johnson, Marc J. A.; Peters, Jonas C.; Odom, Aaron L.; Kim, Esther; Cummins, Christopher C.; George, Graham N.; Pickering, Ingrid J. (1996). "Dinitrogen Cleavage by Three-Coordinate Molybdenum(III) Complexes: Mechanistic and Structural Data1". Journal of the American Chemical Society. 118 (36): 8623–8638. doi:10.1021/ja960574x.
- ^ Dehnicke, Kurt; Weller, Frank; Strähle, Joachim (2001). "Nitrido bridges between transition metals and main group elements illustrated by the series [M]NNa to [M]NCl". Chem. Soc. Rev. 30 (2): 125–135. doi:10.1039/a802635a.
- ^ Smith, Jeremy M. (2014). "Reactive Transition Metal Nitride Complexes". Progress in Inorganic Chemistry Volume 58. Vol. 58. pp. 417–470. doi:10.1002/9781118792797.ch06. ISBN 9781118792797.
{{cite book}}:|journal=ignored (help) - ^ Gladfelter, Wayne L. (1985). Organometallic Metal Clusters Containing Nitrosyl and Nitrido Ligands. Vol. 24. pp. 41–86. doi:10.1016/S0065-3055(08)60413-X. ISBN 9780120311248.
{{cite book}}:|journal=ignored (help)

![[OsNO3]−, isoelectronic with osmium tetroxide.](http://upload.wikimedia.org/wikipedia/commons/thumb/f/f8/OsNO3_anion.png/165px-OsNO3_anion.png)
![[MoNCl4]−, a square pyramidal Mo(VI) complex.](http://upload.wikimedia.org/wikipedia/commons/thumb/2/29/MoNCl4_anion.png/170px-MoNCl4_anion.png)
![[W2(μ-N)Cl10]−, containing two W(VI) centres bridged by a nitrido ligand.](http://upload.wikimedia.org/wikipedia/commons/thumb/8/82/W2NCl10_anion.png/170px-W2NCl10_anion.png)
![[Ir3N(SO4)6(H2O)3]4−, structurally related to basic iron acetate.](http://upload.wikimedia.org/wikipedia/commons/thumb/0/00/Ir3N%28SO4%296aq3.png/128px-Ir3N%28SO4%296aq3.png)