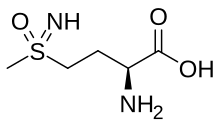
Methionine sulfoximine
| |
| Names | |
|---|---|
| IUPAC name
(2S)-2-Amino-4-(S-methylsulfonimidoyl)butanoic acid
| |
| Other names
l-Methionine sulfoximine; MSO
| |
| Identifiers | |
3D model (JSmol)
|
|
| 1725509 | |
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.016.224 |
| EC Number |
|
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C5H12N2O3S | |
| Molar mass | 180.22 g·mol−1 |
| Related compounds | |
Related compounds
|
Buthionine sulfoximine Glufosinate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Methionine sulfoximine (MSO, also known as MetSox[1]) is an irreversible glutamine synthetase inhibitor. It is the sulfoximine derivative of methionine with convulsant effects.[2]
Methionine sulfoximine is composed of two different diastereomers, which are L-S-Methionine sulfoximine and L-R-Methionine sulfoximine. These affect the longevity of the model mouse for Lou Gehrig's disease.[3] Overproduction of glutamate results to excitotoxicity, which kills the cell. Since methionine sulfoximine inhibits glutamate production in the brain, it prevents excitotoxicity. Thus, increasing the longevity of the mice.[4]
Mechanism of action
[edit]MSO is phosphorylated by glutamine synthetase. The resulting product acts as a transition state analog that is unable to diffuse from the active site, thereby inhibiting the enzyme.[5]
References
[edit]- ^ Carroll, P.; Waddell, S. J.; Butcher, P. D.; Parish, T. (2011). "Methionine sulfoximine resistance in Mycobacterium tuberculosis is due to a single nucleotide deletion resulting in increased expression of the major glutamine synthetase, GlnA1". Microbial Drug Resistance. 17 (3): 351–355. doi:10.1089/mdr.2010.0125. PMC 3161625. PMID 21875360.
- ^ Rowe, WB; Meister, A (June 1970). "Identification of L-methionine-S-sulfoximine as the convulsant isomer of methionine sulfoximine". Proceedings of the National Academy of Sciences of the United States of America. 66 (2): 500–6. Bibcode:1970PNAS...66..500R. doi:10.1073/pnas.66.2.500. PMC 283073. PMID 4393740.
- ^ Brusilow, William S. A. (2017-04-24). "Identification of the isomer of methionine sulfoximine that extends the lifespan of the SOD1 G93A mouse". Neuroscience Letters. 647: 165–167. doi:10.1016/j.neulet.2017.03.029. ISSN 0304-3940. PMID 28323087. S2CID 45664203.
- ^ Bame, Monica; Pentiak, Patricia A.; Needleman, Richard; Brusilow, William S. A. (2012-12-01). "Effect of Sex on Lifespan, Disease Progression, and the Response to Methionine Sulfoximine in the SOD1 G93A Mouse Model for ALS". Gender Medicine. 9 (6): 524–535. doi:10.1016/j.genm.2012.10.014. ISSN 1550-8579. PMID 23217569.
- ^ Krajewski, W. W.; Jones, T. A.; Mowbray, S. L. (18 July 2005). "Structure of Mycobacterium tuberculosis glutamine synthetase in complex with a transition-state mimic provides functional insights". Proceedings of the National Academy of Sciences. 102 (30): 10499–10504. Bibcode:2005PNAS..10210499K. doi:10.1073/pnas.0502248102. PMC 1180770. PMID 16027359.

