K-casein
Κ-casein, or kappa casein, is a mammalian milk protein involved in several important physiological processes. Chymosin (found in rennet) splits K-casein into an insoluble peptide (para kappa-casein) and water-soluble glycomacropeptide (GMP). GMP is responsible for an increased efficiency of digestion, prevention of neonate hypersensitivity to ingested proteins, and inhibition of gastric pathogens.[1] The human gene for κ-casein is CSN3.
Structure
[edit]
Caseins are a family of phosphoproteins (αS1, αS2, β, κ) that account for nearly 80% of bovine milk proteins[3] and that form soluble aggregates are known as "casein micelles" in which κ-casein molecules stabilize the structure. There are several models that account for the spatial conformation of casein in the micelles.[4] One of them proposes that the micellar nucleus is formed by several submicelles, the periphery consisting of microvillosities of κ-casein[5][6] Another model suggests that the nucleus is formed by casein-interlinked fibrils.[7] Finally, the most recent model[8] proposes a double link among the caseins for gelling to take place. All 3 models consider micelles as colloidal particles formed by casein aggregates wrapped up in soluble κ-casein molecules. Milk-clotting proteases act on the soluble portion, κ-casein, thus originating an unstable micellar state that results in clot formation.[9]
Milk clotting
[edit]
Chymosin (EC 3.4.23.4) is an aspartic protease that specifically hydrolyzes the peptide bond in Phe105-Met106 of κ- casein and is considered to be the most efficient protease for the cheesemaking industry.[10] However, there are milk-clotting proteases able to cleave other peptide bonds in the κ-casein chain, such as the endothiapepsin produced by Endothia parasitica.[11] There are also several milk-clotting proteases that, being able to cleave the Phe105-Met106 bond in the κ-casein molecule, also cleave other peptide bonds in other caseins, such as those produced by Cynara cardunculus[6][12][13] or even bovine chymosin.[14] This allows the manufacture of different cheeses with a variety of rheological and organoleptic properties.
The milk-clotting process consists of three main phases:[15]
- Enzymatic degradation of κ-casein.
- Micellar flocculation.
- Gel formation.
Each step follows a different kinetic pattern, the limiting step in milk-clotting being the degradation rate of κ-casein. The kinetic pattern of the second step of the milk-clotting process is influenced by the cooperative nature of micellar flocculation,[16][13] whereas the rheological properties of the gel formed depend on the type of action of the proteases, the type of milk, and the patterns of casein proteolysis.[13] The overall process is influenced by several different factors, such as pH or temperature.[12][9]
The conventional way of quantifying a given milk-clotting enzyme[17] employs milk as the substrate and determines the time elapsed before the appearance of milk clots. However, milk clotting may take place without the participation of enzymes because of variations in physicochemical factors, such as low pH or high temperature.[6][3][9] Consequently, this may lead to confusing and irreproducible results, particularly when the enzymes have low activity. At the same time, the classical method is not specific enough, in terms of setting the precise onset of milk gelation, such that the determination of the enzymatic units involved becomes difficult and unclear. Furthermore, although it has been reported that κ-casein hydrolysis follows typical Michaelis–Menten kinetics,[15] it is difficult to determine with the classic milk-clotting assay.
To overcome this, several alternative methods have been proposed, such as the determination of halo diameter in agar-gelified milk,[17] colorimetric measurement,[18] or determination of the rate of degradation of casein previously labeled with either a radioactive tracer[19] or a fluorochrome compound.[20] All these methods use casein as the substrate to quantify proteolytic or milk-clotting activities.
FTC-Κ-casein assay
[edit]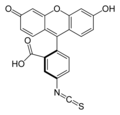
Κ-casein labeled with the fluorochrome fluorescein isothiocyanate (FITC) to yield the fluorescein thiocarbamoyl (FTC) derivative. This substrate is used to determinate the milk clotting activity of proteases.[21]
FTC-κ-casein method affords accurate and precise determinations of κ-caseinolytic degradation, the first step in the milk-clotting process. This method is the result of a modification to the one described by S.S. Twining (1984). The main modification was substituting the substrate previously used (casein) by κ-casein labeled with the fluorochrome fluorescein isothiocyanate (FITC) to yield the fluorescein thiocarbamoyl (FTC) derivative. This variation allows quantification of the κ-casein molecules degraded in a more precise and specific way, detecting only those enzymes able to degrade such molecules. The method described by Twining (1984), however, was designed to detect the proteolytic activity of a considerably larger variety of enzymes. FTC-κ-casein allows the detection of different types of proteases at levels when no milk clotting is yet apparent, demonstrating its higher sensitivity over currently used assay procedures. Therefore, the method may find application as an indicator during the purification or characterization of new milk-clotting enzymes.
Notes
[edit]- ^ "Kappa casein (IPR000117)". InterPro.
- ^ a b Kumosinski, Brown & Farrell 1993.
- ^ a b Lucey, Johnson & Horne 2003.
- ^ Dalgleish 1998.
- ^ Walstra 1979.
- ^ a b c Lucey 2002.
- ^ Holt 1992.
- ^ Horne 1998.
- ^ a b c Vasbinder et al. 2003.
- ^ Rao et al. 1998.
- ^ Drøhse & Foltmann 1989.
- ^ a b Esteves et al. 2003.
- ^ a b c Silva & Malcata 2005.
- ^ Kobayashi 2004.
- ^ a b Carlson, Hill & Olson 1987a.
- ^ Carlson, Hill & Olson 1987b.
- ^ a b Poza et al. 2003.
- ^ Hull 1947.
- ^ Christen 1987.
- ^ Twining 1984.
- ^ Ageitos et al. 2006.
References
[edit]- Ageitos, J.M.; Vallejo, J.A.; Poza, M.; Villa, T.G. (2006). "Fluorescein Thiocarbamoyl-Kappa-Casein Assay for the Specific Testing of Milk-Clotting Proteases". Journal of Dairy Science. 89 (10): 3770–7. doi:10.3168/jds.S0022-0302(06)72418-3. PMID 16960051.
- Carlson, Alfred; Hill, Charles G; Olson, Norman F. (1987). "Kinetics of milk coagulation: I. The kinetics of kappa casein hydrolysis in the presence of enzyme deactivation". Biotechnology and Bioengineering. 29 (5): 582–9. doi:10.1002/bit.260290507. PMID 18576489. S2CID 38359395.
- Carlson, Alfred; Hill, Charles G.; Olson, Norman F. (1987). "Kinetics of milk coagulation: II. Kinetics of the secondary phase: Micelle flocculation". Biotechnology and Bioengineering. 29 (5): 590–600. doi:10.1002/bit.260290508. PMID 18576490. S2CID 44397261.
- Christen, G.L. (1987). "A Rapid Method for Measuring Protease Activity in Milk Using Radiolabeled Casein". Journal of Dairy Science. 70 (9): 1807–14. doi:10.3168/jds.S0022-0302(87)80218-7. PMID 3117854.
- Dalgleish, D.G. (1998). "Casein Micelles as Colloids: Surface Structures and Stabilities". Journal of Dairy Science. 81 (11): 3013–8. doi:10.3168/jds.S0022-0302(98)75865-5.
- Drøhse, Helle B.; Foltmann, Bent (1989). "Specificity of milk-clotting enzymes towards bovine κ-casein". Biochimica et Biophysica Acta (BBA) - Protein Structure and Molecular Enzymology. 995 (3): 221–4. doi:10.1016/0167-4838(89)90039-3. PMID 2495817.
- Esteves, C.L.C.; Lucey, J.A.; Wang, T.; Pires, E.M.V. (2003). "Effect of pH on the Gelation Properties of Skim Milk Gels Made from Plant Coagulants and Chymosin". Journal of Dairy Science. 86 (8): 2558–67. doi:10.3168/jds.S0022-0302(03)73850-8. hdl:10316/3878. PMID 12939079.
- Holt, C. (1992). "Structure and Stability of Bovine Casein Micelles". In Anfinsen, C.B.; Richards, Frederic M.; Edsall, John T.; et al. (eds.). Advances in Protein Chemistry Volume 43. Vol. 43. pp. 63–151. doi:10.1016/S0065-3233(08)60554-9. ISBN 978-0-12-034243-3. PMID 1442324.
- Horne, David S. (1998). "Casein Interactions: Casting Light on the Black Boxes, the Structure in Dairy Products". International Dairy Journal. 8 (3): 171–7. doi:10.1016/S0958-6946(98)00040-5.
- Hull, M.E. (1947). "Studies on Milk Proteins. II. Colorimetric Determination of the Partial Hydrolysis of the Proteins in Milk". Journal of Dairy Science. 30 (11): 881–4. doi:10.3168/jds.S0022-0302(47)92412-0.
- Kobayashi, Hideyuki (2004). "Polyporopepsin". In Barrett, Alan J.; Woessner, J. Fred; Rawlings, Neil D. (eds.). Handbook of Proteolytic Enzymes. pp. 111–5. doi:10.1016/B978-0-12-079611-3.50035-5. ISBN 978-0-12-079611-3.
- Kumosinski, T.F.; Brown, E.M.; Farrell, H.M. (1993). "Three-Dimensional Molecular Modeling of Bovine Caseins: A Refined, Energy-Minimized κ-Casein Structure". Journal of Dairy Science. 76 (9): 2507–20. doi:10.3168/jds.S0022-0302(93)77586-4. PMID 8227653.
- Lucey, J.A. (2002). "Formation and Physical Properties of Milk Protein Gels". Journal of Dairy Science. 85 (2): 281–94. doi:10.3168/jds.S0022-0302(02)74078-2. PMID 11913691.
- Lucey, J.A.; Johnson, M.E.; Horne, D.S. (2003). "Invited Review: Perspectives on the Basis of the Rheology and Texture Properties of Cheese". Journal of Dairy Science. 86 (9): 2725–43. doi:10.3168/jds.S0022-0302(03)73869-7. PMID 14507008.
- Poza, M.; Sieiro, C.; Carreira, L.; Barros-Velázquez, J.; Villa, T. G. (2003). "Production and characterization of the milk-clotting protease of Myxococcus xanthus strain 422". Journal of Industrial Microbiology and Biotechnology. 30 (12): 691–8. doi:10.1007/s10295-003-0100-y. PMID 14634834. S2CID 23067478.
- Rao, Mala B.; Tanksale, Aparna M.; Ghatge, Mohini S.; Deshpande, Vasanti V. (1998). "Molecular and Biotechnological Aspects of Microbial Proteases". Microbiology and Molecular Biology Reviews. 62 (3): 597–635. doi:10.1128/MMBR.62.3.597-635.1998. PMC 98927. PMID 9729602.
- Silva, S.V.; Malcata, F.X. (2005). "Partial Identification of Water-Soluble Peptides Released at Early Stages of Proteolysis in Sterilized Ovine Cheese-Like Systems: Influence of Type of Coagulant and Starter". Journal of Dairy Science. 88 (6): 1947–54. doi:10.3168/jds.S0022-0302(05)72870-8. hdl:10400.14/6738. PMID 15905424.
- Twining, Sally S. (1984). "Fluorescein isothiocyanate-labeled casein assay for proteolytic enzymes". Analytical Biochemistry. 143 (1): 30–4. doi:10.1016/0003-2697(84)90553-0. PMID 6442109.
- Vasbinder, A.J.; Rollema, H.S.; Bot, A.; de Kruif, C.G. (2003). "Gelation Mechanism of Milk as Influenced by Temperature and pH; Studied by the Use of Transglutaminase Cross-Linked Casein Micelles". Journal of Dairy Science. 86 (5): 1556–63. doi:10.3168/jds.S0022-0302(03)73741-2. PMID 12778566.
- Walstra, Pieter (1979). "The voluminosity of bovine casein micelles and some of its implications". Journal of Dairy Research. 46 (2): 317–23. doi:10.1017/S0022029900017234. PMID 469060. S2CID 222355860.
