Mugineic acid
Appearance
| |
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
| ChEBI | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C12H20N2O8 | |
| Molar mass | 320.298 g·mol−1 |
| Appearance | white solid |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
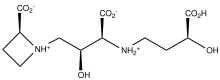
Mugineic acid is the organic compound consisting of a azetidine group and three carboxylates. A colorless solid, it is a siderophore. More specifically, it is a phytosiderophore, i.e. a plant-produced siderophore. It functions as an iron accumulating agent for barley and other plants. Related phytosiderophores include nicotianamine and avenic acid.[1]

It is biosynthesized from S-methylmethionine. The compound binds metal ions as a hexadentate ligand.[4]
References
[edit]- ^ Prasad, Rajendra; Shivay, Yashbir S.; Kumar, Dinesh (2014). Agronomic Biofortification of Cereal Grains with Iron and Zinc. Advances in Agronomy. Vol. 125. pp. 55–91. doi:10.1016/B978-0-12-800137-0.00002-9. ISBN 9780128001370.
- ^ Marsh, Richard E.; Clemente, Dore Augusto (2007). "A survey of crystal structures published in the Journal of the American Chemical Society". Inorganica Chimica Acta. 360 (14): 4017–4024. doi:10.1016/j.ica.2007.02.050.
- ^ Mino, Yoshiki; Ishida, Toshimasa; Ota, Nagayo; Inoue, Masatoshi; Nomoto, Kyosuke; Takemoto, Tsunematsu; Tanaka, Hisashi; Sugiura, Yukio (1983). "Mugineic Acid-Iron(III) Complex and its Structurally Analogous Cobalt(III) Complex: Characterization and Implication for Absorption and Transport of Iron in Gramineous Plants". Journal of the American Chemical Society. 105 (14): 4671–4676. doi:10.1021/ja00352a024.
- ^ Sugiura, Yukio; Tanaka, Hisashi; Mino, Yoshiki; Ishida, Toshimasa; Ota, Nagayo; Inoue, Masatoshi; Nomoto, Kyosuke; Yoshioka, Himeko; Takemoto, Tsunematsu (1981). "Structure, Properties, and Transport Mechanism of Iron(III) Complex of Mugineic Acid, a Possible Phytosiderophore". Journal of the American Chemical Society. 103 (23): 6979–6982. doi:10.1021/ja00413a043.
