Nickel(II) bis(acetylacetonate)
| |

| |
| Names | |
|---|---|
| Other names
Ni(acac)2, nickel acac
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.019.887 |
| EC Number |
|
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C30H42Ni3O12 | |
| Molar mass | 770.734 g·mol−1 |
| Appearance | dark green |
| Density | 1.455 g/cm3 |
| Melting point | 229.5 °C (445.1 °F; 502.6 K) (decomposes) |
| H2O | |
| Hazards | |
| GHS labelling: | |
 
| |
| Danger | |
| H302, H317, H334, H350 | |
| P201, P202, P261, P264, P270, P272, P280, P281, P285, P301+P312, P302+P352, P304+P341, P308+P313, P321, P330, P333+P313, P342+P311, P363, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Nickel(II) bis(acetylacetonate) is a coordination complex with the formula [Ni(acac)2]3, where acac is the anion C5H7O−2 derived from deprotonation of acetylacetone. It is a dark green paramagnetic solid that is soluble in organic solvents such as toluene. It reacts with water to give the blue-green diaquo complex Ni(acac)2(H2O)2.[1]
Structure and properties
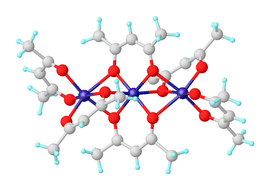
[edit]Anhydrous nickel(II) acetylacetonate exists as molecules of Ni3(acac)6. The three nickel atoms are approximately collinear and each pair of them is bridged by two μ2 oxygen atoms. Each nickel atom has tetragonally distorted octahedral geometry, caused by the difference in the length of the Ni–O bonds between the bridging and non-bridging oxygens.[2] Ni3(acac)6 molecules are almost centrosymmetric, despite the non-centrosymmetric point group of the cis-Ni(acac)2 "monomers," which is uncommon.[3] The trimeric structure allows all nickel centers to achieve an octahedral coordination. The trimer is only formed if intramolecular sharing of oxygen centers between pairs of nickel centers occurs. The anhydrous complex has interesting magnetic properties. Down to about 80 K it exhibits normal paramagnetism with an effective magnetic moment of 3.2 μB, close to the spin-only moment expected of a d8 ion with two unpaired electrons. The effective moment rises to 4.1 μB at 4.3 K, due to ferromagnetic exchange interactions involving all three nickel ions.[4]
When bound to bulkier analogues of acetylacetonate ligand, steric hindrance favors formation of the mononickel derivatives. This behavior is observed for the derivative of 3-methylacetylacetonate.[5]
Dihydrate
[edit]
As in the anhydrous form, the Ni(II) centres occupy octahedral coordination sites. The coordination sphere is provided by two bidentate acetylacetonate (acac) ligands and two aquo ligands. Ni(acac)2(H2O)2 exists as cis and trans isomers.[6] Trans isomers are also observed for Ni(acac)2(pyridine-N-oxide)2.[7] In the trans isomers, the axial Ni–O bonds are greater in length (210.00 pm) than the equatorial Ni–O bonds (200.85 pm and 199.61 pm).[8]

Synthesis
[edit]Bis(2,4-pentanedionato)nickel(II) is prepared by treating nickel nitrate with acetylacetone in the presence of base. The product is the blue-green diaquo complex Ni(CH3COCHCOCH3)2(H2O)2.[9]
- Ni(NO3)2 + 2 CH3COCH2COCH3 + 2 H2O + 2 NaOH → Ni(CH3COCHCOCH3)2(H2O)2 + 2 NaNO3
This complex can be dehydrated using a Dean–Stark trap by azeotropic distillation:[9]
- 3 Ni(CH3COCHCOCH3)2(H2O)2 → [Ni(CH3COCHCOCH3)2]3 + 6 H2O
Upon heating Ni(acac)2(H2O)2 at 170–210 °C under reduced pressure (0.2–0.4 mmHg, 27–53 Pa), the anhydrous form sublimes and water is removed.[3]
Reactions
[edit]The anhydrous complex reacts with a range of Lewis bases to give monomeric adducts.[10] Illustrative is the reaction with tetramethylethylenediamine (tmeda):[11]
- [Ni(CH3COCHCOCH3)2]3 + 3 tmeda → 3 Ni(CH3COCHCOCH3)2(tmeda)
Ni(acac)2(H2O)2 reacts quickly in high yield at a methine positions, producing diamides from isocyanates. Related reactions occur with diethyl azodicarboxylate and dimethyl acetylenedicarboxylate:
- Ni(acac)2(H2O)2 + 2 PhNCO → Ni(O2C5Me2CONHPh)2 + 2 H2O
Applications
[edit]The anhydrous complex is the precursor to nickel-based catalysts such as nickel bis(cyclooctadiene) and tetramethylethylenediamine(dimethyl)nickel(II).[12][11]
[Ni(acac)2]3 is a precursor for the deposition of a thin film of NiO on conductive glass substrates using sol-gel techniques.[10]

See also
[edit]References
[edit]- ^ R. C. Mehrotra; R. Bohra; D. P. Gaur (1978). Metal β-Diketones and Allied Derivatives. Academic Press. ISBN 0124881505.
- ^ G. J. Bullen, R. Mason & P. Pauling (1961). "Octahedral Co-ordination of Nickel in Nickel(II) Bisacetylacetone". Nature. 189 (4761): 291–292. doi:10.1038/189291a0. S2CID 37394858.
- ^ a b G. J. Bullen, R. Mason & P. Pauling. (1965). "The crystal and Molecular Structure of Bis(acetylacetonato)nickel (II)". Inorganic Chemistry. 4 (4): 456–462. doi:10.1021/ic50026a005.
- ^ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. p. 1157. ISBN 978-0-08-037941-8.
- ^ 1. A. Döhring, R. Goddard, P. W. Jolly, C. Krüger, V. R. Polyakov, "Monomer-Trimer Isomerism in 3-Substituted Pentane-2,4-dione Derivatives of Nickel(II)", Inorg. Chemistry 1997, 36, 177–183. doi:10.1021/ic960441c
- ^ M. Kudrat-e-Zahan, Y. Nishida & H. Sakiyama (2010). "Identification of cis/trans isomers of bis(acetylacetonato)nickel(II) complexes in solution based on electronic spectra". Inorganica Chimica Acta. 363: 168–172. doi:10.1016/j.ica.2009.09.011.
- ^ B. N. Figgis; M. A. Hitchman (2000). "Ligand Field Theory and its Application".
{{cite journal}}: Cite journal requires|journal=(help) - ^ O. Metin, L. T. Yildirim & S. Ozkar (2007). "Synthesis, characterization and crystal structure of bis(acetylacetonato)dimethanolnickel(II)". Inorganic Chemistry. 10 (9): 1121–1123. doi:10.1016/j.inoche.2007.06.011.
- ^ a b Wielandt, J. W.; Ruckerbauer, D. (2010). Bis(1,5-cyclooctadiene)nickel(0). Inorganic Syntheses. Vol. 35. p. 120. doi:10.1002/9780470651568.ch6.
- ^ a b Paul A. Williams; Anthony C. Jones; Jamie F. Bickley; Alexander Steiner; Hywel O. Davies; Timothy J. Leedham; Susan A. Impey; Joanne Garcia; Stephen Allen; Aline Rougier; Alexandra Blyr (2001). "Synthesis and Crystal Structures of Dimethylaminoethanol Adducts of Ni(II) Acetate and Ni(II) Acetylacetonate. Precursors for the Sol–Gel Deposition of Electrochromic Nickel Oxide Thin Films". Journal of Materials Chemistry. 11 (9): 2329–2334. doi:10.1039/b103288g.
- ^ a b Kaschube, Wilfried; Pörschke, Klaus R.; Wilke, Günther (1988). "Tmeda-Nickel-Komplexe". Journal of Organometallic Chemistry. 355 (1–3): 525–532. doi:10.1016/0022-328X(88)89050-8.
- ^ Göttker-Schnetmann, Inigo; Mecking, Stefan (2020). "A Practical Synthesis of (tmeda)Ni(CH3)2, Isotopically Labeled (tmeda)Ni(13CH3)2, and Neutral Chelated-Nickel Methyl Complexes". Organometallics. 39 (18): 3433–3440. doi:10.1021/acs.organomet.0c00500. S2CID 224930545.
- ^ Shrestha, Ruja; Dorn, Stephanie C. M.; Weix, Daniel J. (2013-01-16). "Nickel-Catalyzed Reductive Conjugate Addition to Enones via Allylnickel Intermediates". Journal of the American Chemical Society. 135 (2): 751–762. doi:10.1021/ja309176h. PMC 3547151. PMID 23270480.
