Olestra
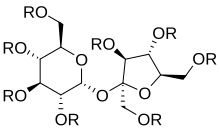 | |
 Top: Generic 2D structure of olestra, where R = H or fatty acid group, C(O)CnHm Bottom: Stereoscopic animation of a representative olestra molecule with 8 unsaturated fatty acid groups | |
| Clinical data | |
|---|---|
| Trade names | Olean |
| Legal status | |
| Legal status |
|
| Identifiers | |
| ChemSpider |
|
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C n+12H 2n+22O 13 (where fatty acids are saturated) |
| Molar mass | Variable |
| | |
Olestra (also known by its brand name Olean) is a fat substitute that adds no metabolizable calories to products. It has been used in the preparation of otherwise high-fat foods, thereby lowering or eliminating their fat content. The Food and Drug Administration (FDA) originally approved olestra for use in the US as a replacement for fats and oils in prepackaged ready-to-eat snacks in 1996,[1] concluding that such use "meets the safety standard for food additives, reasonable certainty of no harm".[2]: 46399 In the late 2000s, olestra lost its popularity due to supposed side effects and has been largely phased out, but products containing the ingredient can still be purchased at grocery stores in some countries.[citation needed] As of 2024, no products are sold in the United States using Olestra.
Commercialization[edit]
Olestra was discovered accidentally by Procter & Gamble (P&G) researchers F. Mattson and R. Volpenhein in 1968 while researching fats that could be more easily digested by premature infants.[3]: 340 In 1971, P&G met with the Food and Drug Administration (FDA) to examine what sort of testing would be required to introduce olestra as a food additive.[3]: 341
During the following tests, P&G noticed a decline in blood cholesterol levels as a side effect of olestra replacing natural dietary fats. Following this potentially lucrative possibility, in 1975, P&G filed a new request with the FDA to use olestra as a "drug", specifically to lower cholesterol levels.[3]: 341 The lengthy series of studies that followed failed, however, to demonstrate the 15% reduction required by the FDA to be approved as a treatment. Further work on Olestra languished.
In 1984, the FDA allowed Kellogg to claim publicly that their high-fiber breakfast cereals were effective in reducing the risk of cancer. P&G immediately started another test series that lasted three years. When these tests were completed, P&G filed for approval as a food additive for up to 35% replacement of fats in home cooking and 75% in commercial uses.[3]: 341
One of the main concerns the FDA had about olestra was it might encourage consumers to eat more of the "top of the pyramid" foods because of the perception of its being healthier. This could result in consumers engaging in over-consumption, thinking the addition of olestra would remove negative consequences.[3]: 339–40 In light of this possibility, approving it as an additive would have meant consumers would be consuming a food with a relatively high amount of an additive, whose long-term health effects were not documented. This made the FDA particularly hesitant to approve the product, as well as the side effects, such as diarrhea, and concern for the loss of fat-soluble vitamins.[3]: 340 In August 1990, P&G narrowed their focus to "savory snacks", potato chips, tortilla chips, crackers and similar foods.
By this point, the original patents were approaching their 1995 expiration. P&G lobbied for an extension, which they received in December 1993. This extension lasted until 25 January 1996.[4] With pressure from P&G, the approval was finally granted on 24 January, one day before the patent expired, automatically extending the patent two years.[4]
At the time of the 1996 ruling, FDA concluded that, "to avoid being misbranded... olestra-containing foods would need to bear a label statement to inform consumers about possible effects of olestra on the gastrointestinal system. The label statement also would clarify that the added vitamins were present to compensate for any nutritional effects of olestra, rather than to provide enhanced nutritional value".[2]: 46364 The FDA later removed the label saying that the "current label does not accurately communicate information to consumers".[2]: 46387 The FDA also agreed with P&G that the "label statement could be misleading and cause consumers of olestra to attribute serious problems to olestra when this was unlikely to be the case".[2]: 46397
Discontinued products[edit]
Olestra was approved by the Food and Drug Administration for use as a food additive in 1996 and was initially used in potato chips under the WOW brand by Frito Lay. In 1998, the first year olestra products were marketed nationally after the FDA's Food Advisory Committee confirmed a judgment it made two years earlier, sales were over $400 million.[3]: 338 By 2000, though, sales slowed to $200 million. P&G abandoned attempts to widen the uses of olestra and sold off its Cincinnati-based factory to Twin Rivers Technologies in February 2002.[4] The WOW chips were rebranded to "Lay's Light" in 2004[5] and were discontinued by 2016.[6]
Pringles Light potato crisps, manufactured by Kellogg's (though at one time a P&G product), used Olean-brand olestra before being discontinued in 2015.[7]
Side effects[edit]
Starting in 1996, an FDA-mandated health warning label reads "This Product Contains Olestra. Olestra may cause abdominal cramping and loose stools. Olestra inhibits the absorption of some vitamins and other nutrients. Vitamins A, D, E, and K have been added".[8]
These symptoms, normally occurring only by excessive consumption in a short period of time, are known as steatorrhea and are caused by an excess of fat in stool.
The FDA removed the warning requirement in 2003, as it had "conducted a scientific review of several post-market studies submitted by P&G, as well as adverse event reports submitted by P&G and the Center for Science in the Public Interest. The FDA concluded the label statement was no longer warranted".[9] The FDA also agreed with P&G that the "label statement could be misleading and cause consumers of olestra to attribute serious problems to olestra when this [was] unlikely to be the case".[2]: 46397
When removing the olestra warning label, the FDA cited a six-week P&G study of more than 3000 people showing the olestra-eating group experienced only a small increase in bowel movement frequency compared to the control group.[10] The FDA concluded that "subjects eating olestra-containing chips were no more likely to report having had loose stools, abdominal cramps, or any other GI symptom compared to subjects eating an equivalent amount of [potato] chips".[2]: 46372
In addition to the effects of the health warnings on public acceptance of the product, olestra might not have lived up to consumer expectations of speedy results. If consumers believed that they could eat more to compensate for the fat calories "saved", olestra would not be an effective way to improve overall diet.[3]: 353 The manufacturers claim that the authentic taste and feel of olestra offsets this tendency,[11] and some studies have shown that people who consume foods with olestra don't eat more to offset the loss in calories.[12] P&G conducted publicity campaigns to highlight olestra's benefits, including working directly with the health-care community.[3]: 351
Olestra is prohibited from sale in many markets, including the European Union and Canada.[13][14]
Consumption of olestra may encourage rats to eat too much of foods containing regular fats, due to the learning of an incorrect association between fat intake and calories. Rats that were fed regular potato chips as well as chips cooked with olestra gained more weight when subsequently eating a high-fat diet than rats that received just regular chips.[15]
Chemistry[edit]
Triglycerides, the energy-yielding dietary fats, consist of three fatty acids bonded in the form of esters to a glycerol backbone. Olestra uses sucrose as the backbone in place of glycerol, and it can form esters with up to eight fatty acids.[16] Olestra is a mixture of hexa-, hepta-, and octa-esters of sucrose with various long chain fatty acids. The resulting radial arrangement is too large and irregular to move through the intestinal wall and be absorbed into the bloodstream. Olestra has the same taste and mouthfeel as fat, but it passes through the gastrointestinal tract undigested without contributing calories or nutritive value to the diet.[17]
From a mechanical point of view, scientists were able to manipulate the compound in such a way that it could be used in place of cooking oils in the preparation of many types of food.[3]: 340
Since it contains fatty acid functional groups, olestra is able to dissolve lipid-soluble vitamins, such as vitamin D, vitamin E, vitamin K, and vitamin A, along with carotenoids. Fat-soluble nutrients consumed with olestra products are excreted with the undigested olestra molecules. To counteract this loss of nutrients, products made with olestra are fortified with oil-soluble vitamins.[18]
Applications[edit]
P&G is marketing its sucrose ester products under the brand "Sefose" for use as an industrial lubricant and paint additive.[19] Because olestra is made by chemically combining sugar and vegetable oil, it releases no toxic fumes and could potentially become a safe and environmentally friendly replacement for petrochemicals in these applications.[20] It is currently used as a base for deck stains and a lubricant for small power tools, and there are plans to use it on larger machinery.[21]
There is preliminary evidence that indicates that administration of olestra may accelerate the excretion of hydrophobic toxins, although there have been no randomized controlled clinical trials to establish the effectiveness of this potential treatment.[22] Toxins that have been studied in conjunction with olestra treatment include dioxins,[23] hexachlorobenzene,[24] and polychlorinated biphenyls (PCBs).[25][26]
References[edit]
- ^ "21CFR172.867: Olestra". Code of Federal Regulations. U.S. Food and Drug Administration.
- ^ a b c d e f "Food Additives Permitted for Direct Addition to Food for Human Consumption; Olestra" (PDF). Federal Register. August 5, 2003. p. 46364 – via govinfo.gov.
- ^ a b c d e f g h i j Nestle M (2013). "Chapter 15: Selling the Ultimate Techno-food: Olestra". Food Politics (10th ed.). London: University of California Press, Ltd. ISBN 978-0-520-27596-6.
- ^ a b c "A Brief History of Olestra". Center for Science in the Public Interest. Archived from the original on December 12, 2003.
- ^ "Frito-Lay Target of Olestra Lawsuit". Consumer Affairs. January 4, 2006. Retrieved May 12, 2021.
- ^ Winderl AM (April 8, 2016). "5 Sneaky Ingredients In Food That Can Cause Diarrhea". SELF. Condé Nast. Retrieved May 12, 2021.
- ^ "@flybrook Sorry for the disappointment! Our Fat Free Pringles have been discontinued. We'll let the team know you would like them back!". Pringles. September 1, 2015. Retrieved May 12, 2021 – via Twitter.
- ^ "FDA approves fat substitute, Olestra". FDA.gov. January 24, 1996. Archived from the original on May 12, 2009. Retrieved December 6, 2006.
- ^ "FDA Changes Labeling Requirement for Olestra". Food and Drug Administration. Archived from the original on May 11, 2009. Retrieved October 12, 2007.
- ^ "Nutrition Facts Label". Frito-Lay North America, Inc. a Division of PepsiCo. Archived from the original on May 11, 2021.
- ^ "Olean Brand Olestra: Frequently Asked Questions". Archived from the original on March 7, 2012. Retrieved April 16, 2012.
- ^ Bray GA, Sparti A, Windhauser MM (March 1995). "Effect of Two Weeks Fat Replacement by Olestra on Food Intake and Energy Metabolism". FASEB J. 9 (3): A439.
- ^ Peale C (June 23, 2000). "Canadian ban adds to woes for P&G's olestra". The Cincinnati Enquirer. Retrieved March 31, 2011.
- ^ "Everything you wanted to know about Olestra". Healthy and Hot. August 23, 2007. Archived from the original on July 11, 2011. Retrieved March 31, 2011.
- ^ Swithers SE, Ogden SB, Davidson TL (August 2011). "Fat substitutes promote weight gain in rats consuming high-fat diets". Behavioral Neuroscience. 125 (4): 512–8. doi:10.1037/a0024404. PMC 3144274. PMID 21688890.
- ^ Orna MV (1993). "Artificial fats, Simplesse, Olestra" (PDF). Food and Chemistry. University of Nebraska-Lincoln. p. 29. Archived from the original (PDF) on May 26, 2011.
- ^ "May Cause Anal Leakage: The Olestra Fat-Free Snack Controversy of the 1990s". April 9, 2020.
- ^ "The Problems With Olestra". Center for Science in the Public Interest. Archived from the original on July 2, 2007.
- ^ "Sefose". P&G Chemicals. Archived from the original on May 9, 2012. Retrieved August 20, 2012.
- ^ Ballantyne C (April 6, 2009). "Olestra makes a comeback – This time in paints and lubricants, not potato chips". 60-Second Science. Scientific American. Archived from the original on April 10, 2009. Retrieved April 12, 2009.
- ^ Ballantyne C (April 13, 2009). "New chemicals for eco-friendly paints and lubricants". 60-Second Science. Scientific American. Archived from the original on April 15, 2009. Retrieved April 14, 2009.
- ^ Klein AV, Kiat H (December 2015). "Detox diets for toxin elimination and weight management: a critical review of the evidence". Journal of Human Nutrition and Dietetics. 28 (6): 675–86. doi:10.1111/jhn.12286. PMID 25522674.
- ^ Geusau A, Abraham K, Geissler K, Sator MO, Stingl G, Tschachler E (August 2001). "Severe 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) intoxication: clinical and laboratory effects". Environmental Health Perspectives. 109 (8): 865–869. doi:10.1289/ehp.01109865. PMC 1240417. PMID 11564625.
- ^ Jandacek RJ, Anderson N, Liu M, Zheng S, Yang Q, Tso P (February 2005). "Effects of yo-yo diet, caloric restriction, and olestra on tissue distribution of hexachlorobenzene". American Journal of Physiology. Gastrointestinal and Liver Physiology. 288 (2): G292–9. doi:10.1152/ajpgi.00285.2004. PMID 15513954. S2CID 3218642.
- Lay summary in: "Olestra Could Be Antidote to Toxins". University of Cincinnati Health News. February 2005. Archived from the original on September 4, 2006.
- ^ Redgrave TG, Wallace P, Jandacek RJ, Tso P (June 2005). "Treatment with a dietary fat substitute decreased Arochlor 1254 contamination in an obese diabetic male". The Journal of Nutritional Biochemistry. 16 (6): 383–4. doi:10.1016/j.jnutbio.2004.12.014. PMID 15936651.
- ^ Jandacek RJ, Heubi JE, Buckley DD, Khoury JC, Turner WE, Sjödin A, et al. (April 2014). "Reduction of the body burden of PCBs and DDE by dietary intervention in a randomized trial". The Journal of Nutritional Biochemistry. 25 (4): 483–8. doi:10.1016/j.jnutbio.2014.01.002. PMC 3960503. PMID 24629911.
