Pentagonal orthobirotunda
| Pentagonal orthobirotunda | |
|---|---|
 | |
| Type | Birotunda, Johnson J33 – J34 – J35 |
| Faces | 2x10 triangles 2+10 pentagons |
| Edges | 60 |
| Vertices | 30 |
| Vertex configuration | 10(32.52) 2.10(3.5.3.5) |
| Symmetry group | D5h |
| Properties | convex |
| Net | |
 | |
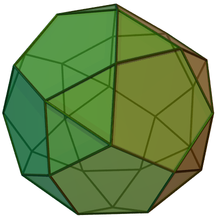
In geometry, the pentagonal orthobirotunda is a polyhedron constructed by attaching two pentagonal rotundae along their decagonal faces, matching like faces. It is an example of Johnson solid.
Construction
[edit]The pentagonal orthobirotunda is constructed by attaching two pentagonal rotundas to their base, covering decagon faces. The resulting polyhedron has 32 faces, 30 vertices, and 60 edges. This construction is similar to icosidodecahedron (or pentagonal gyrobirotunda), an Archimedean solid: the difference is one of its rotundas twisted around 36°, making the pentagonal faces connect to the triangular one, a process known as gyration.[1][2] A convex polyhedron in which all of the faces are regular polygons is the Johnson solid. The pentagonal orthobirotunda is one of them, enumerated as the 34th Johnson solid .[3]
Properties
[edit]The surface area of an icosidodecahedron can be determined by calculating the area of all pentagonal faces. The volume of an icosidodecahedron can be determined by slicing it off into two pentagonal rotunda, after which summing up their volumes. Therefore, its surface area and volume can be formulated as:[1]
References
[edit]- ^ a b Berman, Martin (1971). "Regular-faced convex polyhedra". Journal of the Franklin Institute. 291 (5): 329–352. doi:10.1016/0016-0032(71)90071-8. MR 0290245.
- ^ Ogievetsky, O.; Shlosman, S. (2021). "Platonic compounds and cylinders". In Novikov, S.; Krichever, I.; Ogievetsky, O.; Shlosman, S. (eds.). Integrability, Quantization, and Geometry: II. Quantum Theories and Algebraic Geometry. American Mathematical Society. p. 477. ISBN 978-1-4704-5592-7.
- ^ Francis, Darryl (2013). "Johnson solids & their acronyms". Word Ways. 46 (3): 177.
External links
[edit]







