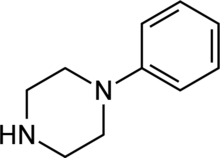
Phenylpiperazine
This article's use of external links may not follow Wikipedia's policies or guidelines. (December 2023) |
| |

| |
| Names | |
|---|---|
| Preferred IUPAC name
1-Phenylpiperazine | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.001.969 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C10H14N2 | |
| Molar mass | 162.23 g/mol |
| Appearance | clear colourless to yellow liquid |
| Density | 1.028g/cm3 |
| Melting point | 18.8 °C (65.8 °F; 291.9 K) |
| Boiling point | 287.2 °C (549.0 °F; 560.3 K) at 760mmHg |
| insoluble | |
| Hazards | |
| Flash point | 138.3 °C (280.9 °F; 411.4 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
1-Phenylpiperazine is a simple chemical compound featuring a phenyl group bound to a piperazine ring. The suffix ‘-piprazole’ is sometimes used in the names of drugs to indicate they belong to this class.[1]
1-Phenylpiperazine is toxic, its oral LD50 in rats is 210 mg/kg.[2]
List phenylpiperazine derivatives[edit]
- Alpertine [27076-46-6]
- BP 554 [82900-57-0]
- Butropipazone [2354-61-2]
- CAM89 alluded to here: WO 2018102233
- Centphenaquin [98459-16-6]
- Centpropazine [91315-34-3] [34675-77-9]
- Clodoxopone [71923-34-7]
- Dropropizine [17692-31-8]
- Etoperidone
- FAUC-299 [313972-96-2]
- FAUC-312 [562104-72-7]
- LASSBio-579 [591774-47-9]
- LASSBio-581 [591774-48-0]
- LASSBio-632
- LASSBio-680
- LASSBio-724
- LASSBio-729 [66307-58-2]
- LASSBio-730
- McN 261 [1044-59-3]
- Nefazodone
- Niaprazine
- Oxypertine
- PD-158771 [189152-50-9]
- PO-219
- Trazodone
- WIN 18,437 [4121-77-1]
See also[edit]
- Substituted piperazine
- Serotonin antagonist and reuptake inhibitor
- Benzylpiperazine
- Diphenylpiperazine
- Diphenylmethylpiperazine
- Pyridinylpiperazine
- Pyrimidinylpiperazine
References[edit]
- ^ World Health Organization (WHO) (2006). "The use of stems in the selection of International Nonproprietary Names (INN) for pharmaceutical substances" (PDF). Archived from the original (PDF) on 2008-12-14. Retrieved 27 April 2010.
- ^ "1-Phenylpiperazine".
External links[edit]
 Media related to Phenylpiperazine at Wikimedia Commons
Media related to Phenylpiperazine at Wikimedia Commons
