From Wikipedia, the free encyclopedia
Chemical compound
Pharmaceutical compound
Quadrosilan Trade names Cisobitan Other names Quadrosilane; KABI-1774; 2,6-cisdiphenylhexa- Drug class Nonsteroidal estrogen
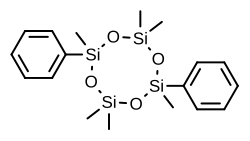
2,2,4,6,6,8-hexamethyl-4,8-diphenyl-1,3,5,7,2,4,6,8-tetraoxatetrasilocane
CAS Number PubChem CID ChemSpider UNII CompTox Dashboard (EPA ) Formula C 18 H 28 O 4 Si 4 Molar mass −1 3D model (JSmol )
C[Si]1(O[Si](O[Si](O[Si](O1)(C)C2=CC=CC=C2)(C)C)(C)C3=CC=CC=C3)C
InChI=1S/C18H28O4Si4/c1-23(2)19-25(5,17-13-9-7-10-14-17)21-24(3,4)22-26(6,20-23)18-15-11-8-12-16-18/h7-16H,1-6H3
Key:ZTQZMPQJXABFNC-UHFFFAOYSA-N
Quadrosilan (INN Tooltip International Nonproprietary Name , BAN Tooltip British Approved Name ) (brand name Cisobitan ; former developmental code name KABI-1774 ) is a synthetic nonsteroidal estrogen that was developed in the 1970s and that is or has been used as an antigonadotropic agent in the treatment of prostate cancer .[ 1] [ 2] [ 3] [ 4] organosilicon compound, and is also known as 2,6-cisdiphenylhexamethylcyclotetrasiloxane .[ 3] [ 5] estrogenic activity equivalent to that of estradiol ,[ 6] feminization and gynecomastia as side effects in male patients.[ 7] [ 8]
^ Elks J (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies ISBN 978-1-4757-2085-3 ^ Morton IK, Hall JM (6 December 2012). Concise Dictionary of Pharmacological Agents: Properties and Synonyms ISBN 978-94-011-4439-1 ^ a b Alfthan O, Andersson L, Esposti PL, Fosså SD, Gammelgaard PA, Gjöres JE, et al. (1983). "Cisobitan in treatment of prostatic cancer. A prospective controlled multicentre study". Scandinavian Journal of Urology and Nephrology . 17 (1): 37–43. doi :10.3109/00365598309179778 . PMID 6346476 . ^ Chisholm GD (1985). "Treatment of advanced cancer of the prostate". Seminars in Surgical Oncology . 1 (1): 38–55. doi :10.1002/ssu.2980010106 . PMID 3887539 . ^ Apeloig Y (1989). The Chemistry of Organic Silicon Compounds ISBN 978-0-471-91993-3 ^ Mills JS, Showell GA (September 2004). "Exploitation of silicon medicinal chemistry in drug discovery". Expert Opinion on Investigational Drugs . 13 (9): 1149–1157. doi :10.1517/13543784.13.9.1149 . PMID 15330746 . S2CID 26669175 . ^ Strindberg B (1978). "Biochemical Effects of 2, 6-cis-Diphenylhexamethylcyclotetrasiloxane in Man". Biochemistry of Silicon and Related Problems . pp. 515–520. doi :10.1007/978-1-4613-4018-8_23 . ISBN 978-1-4613-4020-1 ^ Krarup T, Rasmussen F, Gammelgaard PA (1978). "Prostatic carcinoma treated with 2,6-cis-diphenylhexamethylcyclotetrasiloxane (Cisobitan)". Scandinavian Journal of Urology and Nephrology . 12 (1): 11–15. PMID 345431 .
Estrogens
ER Tooltip Estrogen receptor agonists
Steroidal: Alfatradiol Certain androgens /anabolic steroids (e.g., testosterone , testosterone esters , methyltestosterone , metandienone , nandrolone esters ) (via estrogenic metabolites)
Certain progestins (e.g., norethisterone , noretynodrel , etynodiol diacetate , tibolone )
Clomestrone Cloxestradiol acetate Conjugated estriol Conjugated estrogens Epiestriol Epimestrol Esterified estrogens Estetrol † Estradiol Estradiol esters (e.g., estradiol acetate , estradiol benzoate , estradiol cypionate , estradiol enanthate , estradiol undecylate , estradiol valerate , polyestradiol phosphate , estradiol ester mixtures (Climacteron ))Estramustine phosphate Estriol Estriol esters (e.g., estriol succinate , polyestriol phosphate )Estrogenic substances Estrone Estrone esters
Ethinylestradiol #
Hydroxyestrone diacetate Mestranol Methylestradiol Moxestrol Nilestriol Prasterone (dehydroepiandrosterone; DHEA)
Promestriene Quinestradol Quinestrol Progonadotropins
Antiestrogens
ER Tooltip Estrogen receptor antagonistsSERMs Tooltip selective estrogen receptor modulators /SERDs Tooltip selective estrogen receptor downregulators )Aromatase inhibitors Antigonadotropins
Androgens /anabolic steroids (e.g., testosterone , testosterone esters , nandrolone esters , oxandrolone , fluoxymesterone )D2 receptor antagonists (prolactin releasers) (e.g., domperidone , metoclopramide , risperidone , haloperidol , chlorpromazine , sulpiride )GnRH agonistsleuprorelin , goserelin )GnRH antagonistscetrorelix , elagolix )Progestogens (e.g., chlormadinone acetate , cyproterone acetate , gestonorone caproate , hydroxyprogesterone caproate , medroxyprogesterone acetate , megestrol acetate ) Others
GnRH modulatorsanalogues )
Gonadotropins
Others
Progonadotropins Antigonadotropins
Sex steroid agonists (via negative feedback on the HPG axis Androgens /anabolic steroids (e.g., testosterone , nandrolone esters , oxandrolone )D2 receptor antagonists (prolactin releasers ) (incl., domperidone , metoclopramide , risperidone , haloperidol , chlorpromazine , sulpiride )Estrogens (incl., bifluranol , estradiol , estradiol esters , ethinylestradiol , diethylstilbestrol , paroxypropione )Progestogens (incl. progestins , e.g., chlormadinone acetate , cyproterone acetate , hydroxyprogesterone caproate , gestonorone caproate , medroxyprogesterone acetate , megestrol acetate )
ER Tooltip Estrogen receptor
Agonists
Steroidal: 2-Hydroxyestradiol 2-Hydroxyestrone 3-Methyl-19-methyleneandrosta-3,5-dien-17β-ol 3α-Androstanediol 3α,5α-Dihydrolevonorgestrel 3β,5α-Dihydrolevonorgestrel 3α-Hydroxytibolone 3β-Hydroxytibolone 3β-Androstanediol 4-Androstenediol 4-Androstenedione 4-Fluoroestradiol 4-Hydroxyestradiol 4-Hydroxyestrone 4-Methoxyestradiol 4-Methoxyestrone 5-Androstenediol 7-Oxo-DHEA 7α-Hydroxy-DHEA 7α-Methylestradiol 7β-Hydroxyepiandrosterone 8,9-Dehydroestradiol 8,9-Dehydroestrone 8β-VE2 10β,17β-Dihydroxyestra-1,4-dien-3-one (DHED) 11β-Chloromethylestradiol 11β-Methoxyestradiol 15α-Hydroxyestradiol 16-Ketoestradiol 16-Ketoestrone 16α-Fluoroestradiol 16α-Hydroxy-DHEA 16α-Hydroxyestrone 16α-Iodoestradiol 16α-LE2 16β-Hydroxyestrone 16β,17α-Epiestriol (16β-hydroxy-17α-estradiol) 17α-Estradiol (alfatradiol )17α-Dihydroequilenin 17α-Dihydroequilin 17α-Epiestriol (16α-hydroxy-17α-estradiol) 17α-Ethynyl-3α-androstanediol 17α-Ethynyl-3β-androstanediol 17β-Dihydroequilenin 17β-Dihydroequilin 17β-Methyl-17α-dihydroequilenin Abiraterone Abiraterone acetate Alestramustine Almestrone Anabolic steroids (e.g., testosterone and esters , methyltestosterone , metandienone (methandrostenolone) , nandrolone and esters , many others; via estrogenic metabolites)Atrimustine Bolandiol Bolandiol dipropionate Butolame Clomestrone Cloxestradiol
Conjugated estriol Conjugated estrogens Cyclodiol Cyclotriol DHEA DHEA-S ent -EstradiolEpiestriol (16β-epiestriol, 16β-hydroxy-17β-estradiol) Epimestrol Equilenin Equilin ERA-63 (ORG-37663) Esterified estrogens Estetrol Estradiol
Estramustine Estramustine phosphate Estrapronicate Estrazinol Estriol
Estrofurate Estrogenic substances Estromustine Estrone
Etamestrol (eptamestrol) Ethinylandrostenediol
Ethinylestradiol
Ethinylestriol Ethylestradiol Etynodiol Etynodiol diacetate Hexolame Hippulin Hydroxyestrone diacetate Lynestrenol Lynestrenol phenylpropionate Mestranol Methylestradiol Moxestrol Mytatrienediol Nilestriol Norethisterone Noretynodrel Orestrate Pentolame Prodiame Prolame Promestriene RU-16117 Quinestradol Quinestrol Tibolone Xenoestrogens: Anise -related (e.g., anethole , anol , dianethole , dianol , photoanethole )Chalconoids (e.g., isoliquiritigenin , phloretin , phlorizin (phloridzin) , wedelolactone )Coumestans (e.g., coumestrol , psoralidin )Flavonoids (incl. 7,8-DHF , 8-prenylnaringenin , apigenin , baicalein , baicalin , biochanin A , calycosin , catechin , daidzein , daidzin , ECG , EGCG , epicatechin , equol , formononetin , glabrene , glabridin , genistein , genistin , glycitein , kaempferol , liquiritigenin , mirificin , myricetin , naringenin , penduletin , pinocembrin , prunetin , puerarin , quercetin , tectoridin , tectorigenin )Lavender oil Lignans (e.g., enterodiol , enterolactone , nyasol (cis -hinokiresinol) )Metalloestrogens (e.g., cadmium )Pesticides (e.g., alternariol , dieldrin , endosulfan , fenarimol , HPTE , methiocarb , methoxychlor , triclocarban , triclosan )Phytosteroids (e.g., digitoxin (digitalis ), diosgenin , guggulsterone )Phytosterols (e.g., β-sitosterol , campesterol , stigmasterol )Resorcylic acid lactones (e.g., zearalanone , α-zearalenol , β-zearalenol , zearalenone , zeranol (α-zearalanol) , taleranol (teranol, β-zearalanol) )Steroid -like (e.g., deoxymiroestrol , miroestrol )Stilbenoids (e.g., resveratrol , rhaponticin )Synthetic xenoestrogens (e.g., alkylphenols , bisphenols (e.g., BPA , BPF , BPS ), DDT , parabens , PBBs , PHBA , phthalates , PCBs )Others (e.g., agnuside , rotundifuran ) MixedSERMs Tooltip Selective estrogen receptor modulators ) Antagonists
Coregulator-binding modulators: ERX-11
GPER Tooltip G protein-coupled estrogen receptor
Agonists Antagonists Unknown