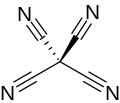
Tetracyanomethane
| |

| |
| Names | |
|---|---|
| Preferred IUPAC name
Methanetetracarbonitrile | |
| Other names
carbon tetracyanide; 2,2-dicyanomalononitrile
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C(CN)4 | |
| Molar mass | 116.083 g·mol−1 |
| Appearance | white crystals |
| Structure | |
| trigonal | |
| R3c | |
a = 9.062, c = 11.625
| |
Lattice volume (V)
|
137.8 Å3 |
Formula units (Z)
|
6 |
| tetrahedron | |
| Thermochemistry[1] | |
Std enthalpy of
formation (ΔfH⦵298) |
−146.2 kcal/mol |
| −616.4 kcal/mol | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Tetracyanomethane or carbon tetracyanide is an organic compound with the chemical formula C(CN)4. It is a percyanoalkane. It is a molecular carbon nitride. The structure can be considered as methane with all hydrogen atoms replaced by cyanide groups. It was first made by Erwin Mayer in 1969.[2][3]
Properties
[edit]Tetracyanomethane is a solid at room temperature. It decomposes over 160 °C without melting, and although it can be in a dilute vapour, no liquid form is known.[2] The molecules of tetracyanomethane have a tetrahedral symmetry (43m or Td). The molecule has C-C distance of 1.484 Å and C-N distance of 1.161 Å in the gas form. In the solid the C≡N bond shortens to 1.147 Å.[3] The C-C bond has a force constant of 4.86×105 dyn/cm which is slightly greater than the C-Cl bond in carbon tetrachloride, but a fair bit weaker than in the tricyanomethanide ion.[4] At pressures over 7 GPa tetracyanomethane starts to polymerize to form a disorganised covalent network solid. At higher pressure the white colour[1] yellows and darkens to black. Over 20 GPa the polymerization is total.[5]
The bulk modulus K0 = 4.4 and its derivative K0' = 18.[5]
Production
[edit]Tetracyanomethane can be made by reacting cyanogen chloride with silver tricyanomethanide.[4]
- ClCN + AgC(CN)3 → C(CN)4 + AgCl
Reactions
[edit]In an acid solution in water tetracyanomethane is hydrolysed to yield tricyanomethanide and ammonium ions along with carbon dioxide. In alkaline solutions tricyanomethanide and cyanate ions are produced.[4]
See also
[edit]- Tricyanomethane (cyanoform)
- Tetraethynylmethane
References
[edit]- ^ a b Barnes, D.S.; Mortimer, C.T.; Mayer, E. (July 1973). "The enthalpy of formation of tetracyanomethane". The Journal of Chemical Thermodynamics. 5 (4): 481–483. Bibcode:1973JChTh...5..481B. doi:10.1016/S0021-9614(73)80095-3.
- ^ a b Mayer, Erwin (1969). "Darstellung und Eigenschaften von Tetracyanmethan". Monatshefte für Chemie. 100 (2): 462–468. doi:10.1007/BF00904089. S2CID 92450428.
- ^ a b Britton, D. (1 July 1974). "The crystal structure of tetracyanomethane, C(CN)4". Acta Crystallographica Section B. 30 (7): 1818–1821. Bibcode:1974AcCrB..30.1818B. doi:10.1107/S0567740874005863.
- ^ a b c Hester, Ronald E.; Lee, Kenneth Michael; Mayer, Erwin (September 1970). "Tetracyanomethane as a pseudo-(carbon tetrahalide)". The Journal of Physical Chemistry. 74 (18): 3373–3376. doi:10.1021/j100712a011.
- ^ a b Keefer, Derek W.; Gou, Huiyang; Wang, Qianqian; Purdy, Andrew; Epshteyn, Albert; Juhl, Stephen J.; Cody, George D.; Badding, John; Strobel, Timothy A. (12 February 2018). "Tetracyanomethane under Pressure: Extended CN Polymers from Precursors with Built-in sp3 Centers". The Journal of Physical Chemistry A. 122 (11): 2858–2863. Bibcode:2018JPCA..122.2858K. doi:10.1021/acs.jpca.7b10729. OSTI 1430339. PMID 29432685.
