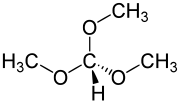
Trimethyl orthoformate
| |

| |
| Names | |
|---|---|
| Preferred IUPAC name
Trimethoxymethane | |
| Other names
2-Methoxyacetaldehyde dimethyl acetal
Methoxymethylal Methyl orthoformate | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.005.224 |
| EC Number |
|
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C4H10O3 | |
| Molar mass | 106.121 g·mol−1 |
| Appearance | Colorless liquid |
| Odor | pungent |
| Density | 0.9676 g/cm3 |
| Melting point | −53 °C (−63 °F; 220 K) |
| Boiling point | 100.6 °C (213.1 °F; 373.8 K) |
| Solubility | soluble in ethanol, diethyl ether |
| Vapor pressure | 1 kPa at 7 °C[2] |
Refractive index (nD)
|
1.3773 |
| Hazards | |
| GHS labelling: | |
 
| |
| Danger | |
| H225, H315, H319, H335 | |
| P210, P233, P240, P241, P242, P243, P261, P264, P271, P280, P302+P352, P303+P361+P353, P304+P340, P305+P351+P338, P312, P321, P332+P313, P337+P313, P362, P370+P378, P403+P233, P403+P235, P405, P501 | |
| Flash point | 13 °C (55 °F; 286 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Trimethyl orthoformate (TMOF) is the organic compound with the formula HC(OCH3)3. A colorless liquid, it is the simplest orthoester. It is a reagent used in organic synthesis for the formation of methyl ethers.[3] The product of reaction of an aldehyde with trimethyl orthoformate is an acetal. In general cases, these acetals can be deprotected back to the aldehyde by using hydrochloric acid.
Synthesis
[edit]Trimethyl orthoformate is prepared on an industrial scale by the methanolysis of hydrogen cyanide:[4]
- HCN + 3 HOCH3 → HC(OCH3)3 + NH3
Trimethyl orthoformate can also be prepared from the reaction between chloroform and sodium methoxide, an example of the Williamson ether synthesis.
Use
[edit]Trimethyl orthoformate is a useful building block for creating methoxymethylene groups and heterocyclic ring systems. It introduces a formyl group to a nucleophilic substrate, e.g. RNH2 to form R-NH-CHO, which can undergo further reactions. It is used in the production of some fungicides (for example: azoxystrobin and picoxystrobin), as well as for some members of the floxacin family of antibacterial drugs.
A number of pharmaceutical intermediates are also made from trimethyl orthoformate.[4]
Trimethyl orthoformate is also an effective reagent for converting compatible carboxylic acids to their corresponding methyl esters.[5] Alternatively, acid-catalyzed esterifications with methanol can be driven closer to completion by employing trimethyl orthoformate to convert water byproduct to methanol and methyl formate.
See also
[edit]- Triethyl orthoformate (triethoxymethane), another trialkyl orthoformate
- Other methoxymethanes
- Methoxymethane (dimethyl ether)
- Dimethoxymethane (methylal)
- Tetramethoxymethane (tetramethyl orthocarbonate)
- Pinner reaction
- Trimethoxysilane, heavier analog, isoelectronic compound
References
[edit]- ^ Trimethyl orthoformate at Sigma-Aldrich
- ^ Alfa Aesar SDS
- ^ Liu, Hui; Tomooka, Craig S.; Xu, Simon L.; Yerxa, Benjamin R.; Sullivan, Robert W.; Xiong, Yifeng; Moore, Harold W. (1999). "Dimethyl Squarate and ITS Conversion to 3-Ethenyl-4-Methoxycyclobutene-1,2-Dione and 2-Butyl-6-Ethenyl-5-Methoxy-1,4-Benzoquinone". Organic Syntheses. 76: 189. doi:10.15227/orgsyn.076.0189.
- ^ a b Ashford's Dictionary of Industrial Chemicals, Third edition, 2011, ISBN 978-0-9522674-3-0, page 9388
- ^ Paine, John B. (1 July 2008). "Esters of Pyromellitic Acid. Part I. Esters of Achiral Alcohols: Regioselective Synthesis of Partial and Mixed Pyromellitate Esters, Mechanism of Transesterification in the Quantitative Esterification of the Pyromellitate System Using Orthoformate Esters, and a Facile Synthesis of the Ortho Pyromellitate Diester Substitution Pattern". The Journal of Organic Chemistry. 73 (13): 4929–4938. doi:10.1021/jo800543w. PMID 18522420.
