User:Jcrosser/sandbox
Arogenate dehydratase[edit]
From Wikipedia, the free encyclopedia
Overview[edit]
|
carboxycyclohexadienyl dehydratase | |
|---|---|
|
Identifiers | |
|
EC number |
4.2.1.91 |
|
Databases | |
|
IntEnz |
IntEnz view |
|
BRENDA |
BRENDA entry |
|
ExPASy |
NiceZyme view |
|
KEGG |
KEGG entry |
|
MetaCyc |
metabolic pathway |
|
PRIAM |
profile |
|
PDBstructures |
RCSB PDB PDBe PDBsum |
|
Gene Ontology |
AmiGO / EGO |
|

In enzymology, an arogenate dehydratase (ADT) (EC 4.2.1.91) is an enzyme that catalyzes the chemical reaction
- L-arogenate → L-phenylalanine + H2O + CO2
Hence, this enzyme has one substrate, L-arogenate, but 3 products: L-phenylalanine, H2O, and CO2. Certain forms of the protein have the potential to catalyze a second reaction,[1]
L-prephenate → L-phenylpyruvate + H2O + CO2
This enzyme participates in phenylalanine, tyrosine, and tryptophan biosynthesis (an example structure is shown to the right[2]).
Nomenclature[edit]
This enzyme belongs to the family of lyases, specifically the hydro-lyases, which cleave carbon-oxygen bonds. The systematic name of this enzyme
class is L-arogenate hydro-lyase
(decarboxylating; L-phenylalanine-forming). Other names in common use include:
- arogenate dehydratase
- L-arogenate hydro-lyase (decarboxylating)
- cyclohexadienyl dehydratase
- carbocyclohexadienyl dehydratase
- pheC
- ADT
Reaction[edit]
The carboxyl and hydroxide groups (shown in red) attached to the 2,5-cyclohexene ring are eliminated from L-arogenate, leaving as carbon dioxide and water. The 2,5-cyclohexene ring becomes a phenyl ring, and L-phenylalanine is formed.
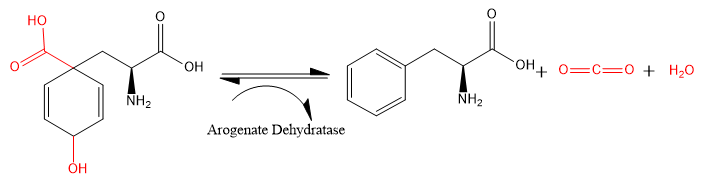
Certain forms of ADT have been shown to exhibit some prephenate dehydratase (PDT) activity in addition to the standard ADT activity described above.[1] Known as cyclohexadienyl dehydratases or carbocyclohexadienyl dehydratases (listed above),[1] these forms of the enzyme catalyze the same type of reaction (a decarboxylation and a dehydration) on prephenate. The carboxyl and hydroxide groups (in red) attached to the 2,5-cyclohexene ring are removed, leaving phenylpyruvate.

Function[edit]
ADT catalyzes a reaction categorized by two major changes in the structure of the substrate, these being a decarboxylation and a dehydration; the enzyme removes a carboxyl group and a water molecule (respectively).[1] Both potential products of this reaction (L-arogenate and phenylpyruvate) occur at or near the end of the biosynthetic pathway.
Structure[edit]
The structure of arogenate dehydratases are described as having, for the most part, three major sections. ADT's contain an N-terminal transit peptide, a PDT-like domain, and an ACT (Aspartokinase, chorismate mutase, TyrA) domain.[3]
Homologues[edit]
Homologues for ADT have been isolated in Arabidopsis thaliana (rabbit-ear cress),[3] Nicotiana sylvestris (tobacco)[4], Spinacia oleracea (spinach)[4], Petunia hybrida,[5] Sorghum bicolor[6], and Oryza sativa[7], which are all considered higher-order plants. Erwinia herbicola[8] and Pseudomonas aeruginosa[9] are known to have homologues for cyclohexadienyl dehydratase. Of the plants with ADT homologues, both Arabidopsis thaliana and Petunia hybrida are known to have paralogues for the gene (six and three, respectively).[3][5]
References[edit source | editbeta][edit]
- ^ a b c d Fischer R, Jensen R (1987). "Arogenate dehydratase". Methods Enzymol. 142: 495–502. doi:10.1016/S0076-6879(87)42061-2. PMID 3600377.
- ^ Tan K, Marshall N, Buck K, Joachimiak A (To be published). "The crystal structure of cyclohexadienyl dehydratase precursor from Pseudomonas aeruginosa PA01."
- ^ a b c Cho MH, Corea ORA, Yang H, Bedgar DL, Laskar DD, et al (2007). "Phenylalanine biosynthesis in Arabidopsis thaliana-- identification and characterization of arogenate dehydratases." J. Biol. Chem. 282: 30827-35.
- ^ a b Jung E, Zamir LO, Jensen RA (1986). "Chloroplasts of higher plants synthesize L-phenylalanine via L-arogenate." Proc. Nat. Acad. Sci. 83 (no. 19): 7231-7235.
- ^ a b Maeda H, Shasany AK, Schnepp J, Orlova I, Taguchi G, et al (2010). "RNAi suppression of Arogenate Dehydratase1 reveals that phenylalanine is synthesized predominantly via the arogenate pathway in petunia petals." Plant Cell. 22: 832-849.
- ^ Siehl DL, Conn EE (1988). "Kinetic and regulatory properties of arogenate dehydratase in seedlings of Sorghum bicolor (L.) Moench". Arch. Biochem. Biophys. 260 (2): 822–9. doi:10.1016/0003-9861(88)90513-9. PMID 3124763.
- ^ Yamada T, Matsuda F, Kasai K, Fukuoka S, Kitamaru K, et al (2008). "Mutation of a rice gene encoding a phenylalanine biosynthetic enzyme results in accumulation of phenylalanine and tryptophan. Plant Cell. 20: 1316-1329
- ^ Xia T, Ahmad S, Zhao G, Jensen RA (1991). "A single cyclohexadienyl dehydratase specifies the prephenate dehydratase and arogenate dehydratase components of two independent pathways to L-phenylalanine in Erwinia herbicola." Arch. Biochem. Biophys. 286: 461-465. PMID 1897969.
- ^ Zhao G, Xia T, Fischer RS, Jensen RA (1992). "Cyclohexadienyl dehydratase from Pseudomonas aeruginosa. Molecular cloning of the gene and characterization of the gene product." J. Biol. Chem. 267: 2487-2493. PMID 1733946.
