User:Mjbailey/bromodomain
Appearance
| Bromodomain | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
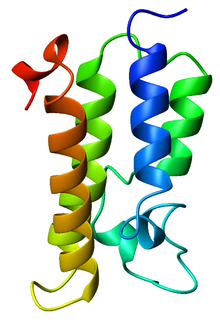 Ribbon diagram of the GCN5 bromodomain from Saccharomyces cerevisiae, colored from blue (N-terminus) to red (C-terminus).[1] | |||||||||||
| Identifiers | |||||||||||
| Symbol | Bromodomain | ||||||||||
| Pfam | PF00439 | ||||||||||
| InterPro | IPR001487 | ||||||||||
| SMART | SM00297 | ||||||||||
| PROSITE | PDOC00550 | ||||||||||
| SCOP2 | 1b91 / SCOPe / SUPFAM | ||||||||||
| CDD | cd04369 | ||||||||||
| |||||||||||
A bromodomain is a protein domain that recognizes acetylated lysine residues such as those on the N-terminal tails of histones. This recognition is often a prerequisite for protein-histone association and chromatin remodeling. The domain itself adopts an all-α protein fold, a bundle of four alpha helices.[1][2]
Discovery[edit]
The bromodomain was identified as a novel structural motif by John W. Tamkun and colleagues studying the brm gene, and showed sequence similarity to genes involved in transcriptional activation.[3]
See also[edit]
References[edit]
- ^ a b PDB: 1e6i; Owen DJ, Ornaghi P, Yang JC, Lowe N, Evans PR, Ballario P, Neuhaus D, Filetici P, Travers AA (November 2000). "The structural basis for the recognition of acetylated histone H4 by the bromodomain of histone acetyltransferase gcn5p". EMBO J. 19 (22): 6141–9. doi:10.1093/emboj/19.22.6141. PMC 305837. PMID 11080160.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Zeng L, Zhou MM (February 2002). "Bromodomain: an acetyl-lysine binding domain". FEBS Lett. 513 (1): 124–8. doi:10.1016/S0014-5793(01)03309-9. PMID 11911891.
- ^ Tamkun JW, Deuring R, Scott MP, Kissinger M, Pattatucci AM, Kaufman TC, Kennison JA (February 1992). "brahma: a regulator of Drosophila homeotic genes structurally related to the yeast transcriptional activator SNF2/SWI2". Cell. 68 (3): 561–72. doi:10.1016/0092-8674(92)90191-E. PMID 1346755.
{{cite journal}}: CS1 maint: multiple names: authors list (link)
