User:Mm9656/sandbox
 | This is a user sandbox of Mm9656. You can use it for testing or practicing edits. This is not the sandbox where you should draft your assigned article for a dashboard.wikiedu.org course. To find the right sandbox for your assignment, visit your Dashboard course page and follow the Sandbox Draft link for your assigned article in the My Articles section. |
A condensation reaction is a class of an organic addition reaction that proceeds in a step-wise fashion to produce the addition product, usually in equilibrium, and a water molecule (hence the name condensation). The reaction may otherwise involve the formation of ammonia, ethanol, or acetic acid[1]. It is a versatile class of reactions that can occur in acidic or basic conditions or in the presence of a catalyst. This class of reactions is a vital part of life as it is essential to the formation of peptide bonds between amino acids[2].

There are also copious variations of condensation reactions carried out in the lab, common examples include the Aldol Condensation, the Claisen Condensation, the Knoevenagel Reaction and the Dieckman Condensation (intramolecular Claisen Condensation)[3].

- ^ "Condensation Reaction". IUPAC Copendium of Chemical Terminology (Gold Book). IUPAC. Retrieved 7 December 2017.
- ^ Voet, Donald; Voet, Judith; Pratt, Chriss (2008). Fundamentals of Biochemistry. Hoboken, NJ: John Wiley & Sons, Inc. p. 88. ISBN 978-0470-12930-2.
- ^ Bruckner, Reinhard (2002). Advanced Organic Chemistry (First ed.). San Diego, California: Harcourt Academic Press. pp. 414–427. ISBN 0-12-138110-2.
{{cite book}}:|access-date=requires|url=(help)
Mechanistic Details
The numerous amount of condensation reactions produce numerous mechanisms but they all tend to follow a similar pathway.
Knoevenagel Reaction Mechanism
This reaction takes between a carbonyl compound and a activated methylene compound or with a nitromethane group, in mildly basic conditions. The final product of the reaction is an alkene with two geminal acceptor groups or one nitro group[1]
![[2]Mechanism for the reaction of active-methylene compounds with carbonyls to form alkenes.](http://upload.wikimedia.org/wikipedia/commons/thumb/9/9f/Knoevenagel_Reaction.png/411px-Knoevenagel_Reaction.png)
Crossed Claisen Condensation Reaction Mechanism
The crossed claisen reaction results in the acylation of an ester enolate with another ester. These reactions are only possible when one ester has no alpha hydrogens. The reaction conditions call for a highly basic solution and a rapid acidic workup to achieve the final compound.
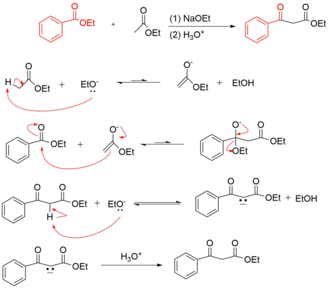
Modern Synthetic Applications
Condensation reactions are used throughout the field of synthetic and medicinal chemistry to synthesize target compounds. Their familiarity and predictable nature make them a widely used synthetic tool. In this section are several examples of some recent uses of condensation reactions in the fields of medicinal and synthetic chemistry.
A team of chemists led by Young Lok Choi and Jung-Nyoung Heo successfully synthesized dibenzoheptenones by combining a suzuki-miyaura coupling reaction with an aldol condensation reaction.[3] These dibenzoheptenone form the skeleton for important medicinal drugs such as colchicine (anti-inflamatory used to treat gout).
Condensation reactions have also recently been used to initiate tandem C-N bond formation to form heterocycles. In a paper published in 2017 by a team led by Yan-Xiao Jiao and Gui-Fa Su* they managed to form quinoxilines which is a structure that is used throughout the pharmaceutical and technological industry.[4]
- ^ Cite error: The named reference
:0was invoked but never defined (see the help page). - ^ Bruckner, Reinhard (2002). Advanced Organic Chemistry. San Diego, California: Harcourt Academic Press. p. 418. ISBN 0-12-138110-2.
- ^ Kim, Joa; Kim, Young; Jung-Nyoung, Heo (Winter 2017). "Total Synthesis of Aristolactams via a One-Pot Suzuki−Miyaura Coupling/Aldol Condensation Cascade Reaction". Journal of Organic Letters. 10: 3543–3546.
- ^ Jiao, Yan-Xiao; Wu, Ling-Ling; Su, Gui-FA (Winter 2017). "Tandem C–N Bond Formation through Condensation and Metal-Free N-Arylation: Protocol for Synthesizing Diverse Functionalized Quinoxalines". Journal of Organic Chemistry. 82: 4407–4417.
