User:Mr. Ibrahem/Carisoprodol
 | |
| Clinical data | |
|---|---|
| Trade names | Soma, others[1] |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682578 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By mouth |
| Drug class | Carbamate |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Protein binding | 60% |
| Metabolism | Liver (CYP2C19-mediated) |
| Metabolites | Meprobamate |
| Onset of action | Rapid |
| Elimination half-life | 2.5 hours |
| Excretion | Kidney |
| Identifiers | |
| |
| Chemical and physical data | |
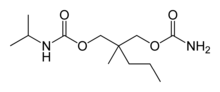
| Formula | C12H24N2O4 |
| Molar mass | 260.334 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Carisoprodol, sold under the brand name Soma among others, is a medication used for musculoskeletal pain.[2] Use is only approved for up to three weeks.[2] Effects generally begin within half an hour and last for up to six hours.[2] It is taken by mouth.[2]
Common side effects include headache, dizziness, and sleepiness.[2] Serious side effect may include addiction, allergic reactions, and seizures.[2] In people with a sulfa allergy certain formulations may result in problems.[2] Safety during pregnancy and breastfeeding is not clear.[4][2] How it works is not clear.[2] Some of its effects are believed to occur following being converted into meprobamate.[2]
Carisoprodol was approved for medical use in the United States in 1959.[2] Its approval in Europe was withdrawn in 2008.[5] It is available as a generic medication.[2] In the United States the wholesale cost is less than US$0.10 per dose.[6] In 2017, it was the 255th most commonly prescribed medication in the United States, with more than one million prescriptions.[7][8] In the United States, it is a Schedule IV controlled substance.[2]
References[edit]
- ^ "Carisoprodol". drugs.com. Archived from the original on 31 August 2017. Retrieved 16 April 2017.
- ^ a b c d e f g h i j k l m n o "Carisoprodol Monograph for Professionals". Drugs.com. American Society of Health-System Pharmacists. Archived from the original on 18 June 2019. Retrieved 8 April 2019.
- ^ "WHOCC - ATC/DDD Index". www.whocc.no. Archived from the original on 10 June 2020. Retrieved 8 September 2020.
- ^ "DailyMed - carisoprodol tablet". dailymed.nlm.nih.gov. Archived from the original on 24 November 2020. Retrieved 8 April 2019.
- ^ "Carisoprodol". European Medicines Agency. 15 November 2007. Archived from the original on 8 April 2019. Retrieved 8 April 2019.
- ^ "NADAC as of 2019-02-27 | Data.Medicaid.gov". Centers for Medicare and Medicaid Services. Archived from the original on 6 March 2019. Retrieved 8 April 2019.
- ^ "The Top 300 of 2020". ClinCalc. Archived from the original on 12 February 2021. Retrieved 11 April 2020.
- ^ "Carisoprodol - Drug Usage Statistics". ClinCalc. Archived from the original on 8 July 2020. Retrieved 11 April 2020.
