From Wikipedia, the free encyclopedia
Chemical compound
Pharmaceutical compound
Vinylbital Routes of Oral ATC code Legal status Metabolism Hepatic Excretion Renal
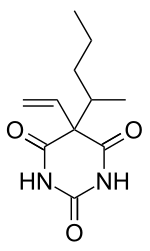
5-(1-methylbutyl)-5-vinylpyrimidine-2,4,6(1H ,3H ,5H )-trione
CAS Number PubChem CID ChemSpider UNII KEGG CompTox Dashboard (EPA ) ECHA InfoCard 100.017.633 Formula C 11 H 16 N 2 O 3 Molar mass −1 3D model (JSmol )
O=C1NC(=O)NC(=O)C1(\C=C)C(C)CCC
InChI=1S/C11H16N2O3/c1-4-6-7(3)11(5-2)8(14)12-10(16)13-9(11)15/h5,7H,2,4,6H2,1,3H3,(H2,12,13,14,15,16)
Y Key:KGKJZEKQJQQOTD-UHFFFAOYSA-N
Y N Y (what is this?) (verify)
Vinylbital , also known as butylvinal , is a sedative hypnotic drug which is a barbiturate derivative.[ 2] [ 3] [failed verification
^ Anvisa (2023-03-31). "RDC Nº 784 - Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial" [Collegiate Board Resolution No. 784 - Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control] (in Brazilian Portuguese). Diário Oficial da União (published 2023-04-04). Archived from the original on 2023-08-03. Retrieved 2023-08-16 .^ Breimer DD, de Boer AG (1976). "Pharmacokinetics and relative bioavailability of vinylbital in man after oral and rectal administration". Arzneimittel-Forschung . 26 (3): 448–54. PMID 989344 . ^ US 2868790 , Brandstrom AE, "Manufacture of Barbituric Acid Compounds", issued 13 January 1959, assigned to Pharmacia
Alcohols Barbiturates Benzodiazepines Carbamates Flavonoids Imidazoles Kava constituentsMonoureides Neuroactive steroids Nonbenzodiazepines Phenols Piperidinediones Pyrazolopyridines Quinazolinones Volatiles /gases Others/unsorted
3-Hydroxybutanal α-EMTBL AA-29504 Alogabat Avermectins (e.g., ivermectin )Bromide compounds (e.g., lithium bromide , potassium bromide , sodium bromide )Carbamazepine Chloralose Chlormezanone Clomethiazole Darigabat DEABL Deuterated etifoxine Dihydroergolines (e.g., dihydroergocryptine , dihydroergosine , dihydroergotamine , ergoloid (dihydroergotoxine) )DS2 Efavirenz Etazepine Etifoxine Fenamates (e.g., flufenamic acid , mefenamic acid , niflumic acid , tolfenamic acid )Fluoxetine Flupirtine Hopantenic acid KRM-II-81 Lanthanum Lavender oil Lignans (e.g., 4-O-methylhonokiol , honokiol , magnolol , obovatol )Loreclezole Menthyl isovalerate (validolum) Monastrol Niacin Niacinamide Org 25,435 Phenytoin Propanidid Retigabine (ezogabine) Safranal Seproxetine Stiripentol Sulfonylalkanes (e.g., sulfonmethane (sulfonal) , tetronal , trional )Terpenoids (e.g., borneol )Topiramate Valerian constituents (e.g., isovaleric acid , isovaleramide , valerenic acid , valerenol )