Wikipedia:Reference desk/Archives/Science/2010 March 17
| Science desk | ||
|---|---|---|
| < March 16 | << Feb | March | Apr >> | March 18 > |
| Welcome to the Wikipedia Science Reference Desk Archives |
|---|
| The page you are currently viewing is an archive page. While you can leave answers for any questions shown below, please ask new questions on one of the current reference desk pages. |
March 17
[edit]Chocolate/Theobromine poisoning
[edit]I know that chocolate (and a few other things containing "Theobromine") is poisonous to dogs and cats (also to horses, parrots and voles). It's not poisonous to humans except in pretty large quantities. Theobromine poisoning doesn't tell us much more than that. How far from humans can a mammal be (from an evolutionary/genetic perspective) and still be able to eat the stuff? Are humans unique? How about chimps? What is the evolutionary benefit to us? It's hard to imagine an evolutionary pressure to survive eating chocolate, tea, cola and Açaí Palm berries. Is there some other metabolism that requires theobromine tolerance? SteveBaker (talk) 01:11, 17 March 2010 (UTC)
- So the article claims
- A typical 20 kg (44 lb) dog will normally experience intestinal distress after eating less than 240 g (8.5 oz) of dark chocolate
- Now, if you scale that up to my weight, I'm pretty sure that would cause me intestinal distress, too. Maybe the risk to dogs is overstated? Or is it more that dogs, who evolutionarily were opportunistic feeders, are more likely to actually eat half a pound of chocolate? --Trovatore (talk) 01:14, 17 March 2010 (UTC)
- But look at the "oral toxicity" chart at the top-right. Humans can tolerate twice as much theobromine per kg of body weight as a dog - and we can metabolize it much faster than they can. In dogs, the dosage is cumulative over days - in humans it's barely cumulative at all. I think there is some significant biochemical difference here. SteveBaker (talk) 01:44, 17 March 2010 (UTC)
- Chemically, theobromine is almost identical to caffeine, and AFAIK has a similar effect on the body. The difference between the two is a single methyl group off one of the nitrogens (caffeine has it, theobromine doesn't). Given my rather limited understanding of alkaloid metabolism, I'm not sure there's fundementally much difference as to how your body would process these chemicals; they form a "class" much as morphine-derivative opiates do, like codeine, morphine, hydrocodone, vicodin, etc. etc. do. In fact, looking at the them, it looks like the difference between codeine and morphine is almost exactly the same as the difference between theobromine and caffeine (1 methyl group) and our article on codeine mentions in the pharmacology section that the body processes codeine into morphine, however only at about a 5-10% efficiency rate, which is why codeine is slower acting and less addictive than morphine. I wonder if a similar relationship exists between caffeine and theobromine; in that case the methylated version (caffeine) would be slower acting by analogy, which would mean that theobromine would be somewhat more dangerous gram-for-gram. However, this is all a giant WAG, as the human body can behave rather unpredictably in these areas, so one would actually need to look for throbromine pharmacology and pharmacokinetics studies. --Jayron32 05:29, 17 March 2010 (UTC)
- According to caffeine, 12% of that compound is metabolized into theobromine, the rest goes down different pathways. They all have some similar effects but the pathways do not appear to reconverge at a later pharmacologically important point prior to excretion. DMacks (talk) 08:44, 17 March 2010 (UTC)
- So that does match my intuition regarding the similarity in relationship between caffeine/theobromine and codeine/morphine, at least on the methylated version metabolizing into the non-methylated one, and at roughly the same rate. However, as DMacks notes, it does appear that beyond that point, metabolicly they are treated differently. That does not necessarily mean that neurologically they are, however, or toxicogically. --Jayron32 14:37, 17 March 2010 (UTC)
- According to caffeine, 12% of that compound is metabolized into theobromine, the rest goes down different pathways. They all have some similar effects but the pathways do not appear to reconverge at a later pharmacologically important point prior to excretion. DMacks (talk) 08:44, 17 March 2010 (UTC)
- Let me offer a hypothesis for the evolutionary difference. People, being omnivores, eat a wide variety of foods, some of which include theobromine. And, at times, they may exclusively eat such foods. Therefore, a tolerance for theobromine would be evolutionarily selected for. Most other animals have a more restricted diet. Thus, unless this restricted diet includes food items heavy in theobromine, there would be little evolutionary pressure for them to develop this tolerance. It would be interesting to check if other omnivores, such as bears, also have a tolerance. StuRat (talk) 13:58, 17 March 2010 (UTC)
- If you're planning on doing some OR with a Hershey bar and a grizzly, please let us know how you make out. Matt Deres (talk) 16:57, 17 March 2010 (UTC)
- If dogs were actually carnivores, that would make sense. In my experience, they are only slightly more selective than goats when it comes to food. Might be interesting to see if there was a difference in the tolerance between dogs and wolves; have 100,000 years of living near humans and stealing (or being fed) human food changed dogs' digestion? —ShadowRanger (talk|stalk) 18:00, 17 March 2010 (UTC)
Galaxies as iron filings
[edit]
If all the Galaxies in the Universe were viewed as little iron filing with the axis perpendicular to the Galactic plan where the clockwise side was the North pole and the Universe viewed as a giant magnet or coil how would the little Galaxy iron filings line up and in what direction would they point? 71.100.11.118 (talk) 01:27, 17 March 2010 (UTC)
- You don't have to guess - look at the famous Hubble Deep Field photograph. The axes of the other galaxies are not parallel to ours - they are oriented in all possible directions...seemingly at random. File:2MASS LSS chart-NEW Nasa.jpg is a picture that shows the large-scale structure of the universe around us - and it seems that galaxies are not evenly distributed - they are mostly grouped into vast strands. However, don't read anything into this in terms of our galaxy somehow influencing the other magnetically or anything - our galaxy is just one of a zillion others. SteveBaker (talk) 01:38, 17 March 2010 (UTC)
- The galaxies seem to be orientated randomly. You might find Large-scale structure of the cosmos interesting. Astronaut (talk) 01:57, 17 March 2010 (UTC)
- It is unlikely that galaxy-sized objects have any significant net electromagnetic properties. To my knowledge, no evidence of a galaxy-sized magnetosphere has ever been observed - it is not even clear what evidence could possibly exist to establish or discredit the idea of a galaxy-scale magnetic field alignment. Galactic magnetic fields seems to be a nice overview of the debate; and here is an IOP article about radio measurements of magnetism in the outer galaxy. Nimur (talk) 02:03, 17 March 2010 (UTC)
- Here is an overview of a Very Large Array experiment to place an upper-bound on the magnitude of an interstellar / inter-galactic magnetic field. Its result - evidence for a very weak field (presented at a conference, not a peer-reviewed journal). Nimur (talk) 02:08, 17 March 2010 (UTC)
- I am referring to magnetism only in the context of it being polar force where alignment along the lines of force in the magnetic field is obvious. Now drop the notion of magnetism and consider the force of gravity instead. 71.100.11.118 (talk) 03:18, 17 March 2010 (UTC)
- Some work in the 1970s seems to indicate that inside a galaxy, certain structures align parallel to the galactic plane - see The alignment of interstellar dust clouds and the differential z-field of the galaxy, for example. However, this is not the entire galaxy aligning with respect to something in the universe - the alignment of individual galaxies appears to be random, and their position appears to be in the form of "strands" or superclusters. Nimur (talk) 03:46, 17 March 2010 (UTC)
- I'm referring to the central perpendicular galactic axis alignment and not to the alignment of matter within the Galaxy with the central perpendicular galactic axis. Although many things look random in nature (and in the presence of dark matter and energy throughout the Universe) you can not tell for sure just by looking. Instead you have to measure the angles of all the central perpendicular axis and determine what points in the Universe their imaginary projections intersect in order to know for sure. 71.100.11.118 (talk) 06:13, 17 March 2010 (UTC)
- Valid point. I was not able to find any reputable source indicating that any alignment exists, even statistically, for all the galactic axes we have observed. If you think such an alignment does exist, you should find some evidence to support that belief; very likely, somebody else has studied this problem, and if there were a conclusion to be drawn, it's probably published in a reputable scientific journal somewhere. Nimur (talk) 06:24, 17 March 2010 (UTC)
- It's remotely possible that some kind of universal "directional influence" exists for galaxies - but if it's there, it has to be extremely subtle because just a quick glance at the Hubble deep field photo is enough to tell you that the axes of the galaxies are all over the place. In just that tiny patch of the photo that I posted (above), you can see galaxies that are everywhere from edge-on to full-on - and that the edge-on ones come in all kinds of rotations compared to the 'vertical' axis of the photo. So if there is some kind of aligning effect, it must be something exceedingly subtle and statistical in nature or we'd see it in the Hubble photo. I think another problem for this idea is that galaxies are not rigid bodies - they are vast collections of individual stars. If some 'aligning force' existed, it wouldn't turn the axes of rotation towards some universal direction without also messing with the shape of the galaxy under its rotation. Hence, there wouldn't be any circular galaxies unless they happened to start off spinning with the right axis. We'd see that galaxies that don't lie in the "universal direction" would be warped...and again, there is no sign of that happening. So given that (a) we have no idea what kind of a force could do this and (b) there is no sign of this alignment happening in practice - then Occam's razor says that we need not concern ourselves overly much with this issue. SteveBaker (talk) 12:33, 17 March 2010 (UTC)
- Don't be quite so quick to step from the deep field photos to "no". In any given direction, the deep field photograph extends through quite a lot of space (à la optical double), so it's possible that we see galaxies with all alignments because we're looking through a variety of non-aligned "field lines" in every direction. However, you're quite right that there's currently no reason to read that much complexity into the image. --Tardis (talk) 13:15, 17 March 2010 (UTC)
- Well, if we have to get into details - there have actually been at least four similar studies done by the Hubble (Extended Groth Strip, Hubble Deep Field South, Hubble Ultra Deep Field and Hubble Deep Field) - each looking in a different direction. The express reason for doing at least two of those studies was specifically in order to verify the "Cosmological principle" - that the universe is homogeneous on large scales. The redshifts of the galaxies imaged are known - so their distances are known. If there were some alignment of galactic axes (which would be a violation of the cosmological principle), it would certainly have been noticed - even if the nature of that alignment were a function of distance or direction. In fact, they did notice a change in the shapes of galaxies that are very far away which can be traced to the fact that such distant galaxies were still quite young when the light that Hubble captured left them. SteveBaker (talk) 02:39, 18 March 2010 (UTC)
- Don't be quite so quick to step from the deep field photos to "no". In any given direction, the deep field photograph extends through quite a lot of space (à la optical double), so it's possible that we see galaxies with all alignments because we're looking through a variety of non-aligned "field lines" in every direction. However, you're quite right that there's currently no reason to read that much complexity into the image. --Tardis (talk) 13:15, 17 March 2010 (UTC)
- It's remotely possible that some kind of universal "directional influence" exists for galaxies - but if it's there, it has to be extremely subtle because just a quick glance at the Hubble deep field photo is enough to tell you that the axes of the galaxies are all over the place. In just that tiny patch of the photo that I posted (above), you can see galaxies that are everywhere from edge-on to full-on - and that the edge-on ones come in all kinds of rotations compared to the 'vertical' axis of the photo. So if there is some kind of aligning effect, it must be something exceedingly subtle and statistical in nature or we'd see it in the Hubble photo. I think another problem for this idea is that galaxies are not rigid bodies - they are vast collections of individual stars. If some 'aligning force' existed, it wouldn't turn the axes of rotation towards some universal direction without also messing with the shape of the galaxy under its rotation. Hence, there wouldn't be any circular galaxies unless they happened to start off spinning with the right axis. We'd see that galaxies that don't lie in the "universal direction" would be warped...and again, there is no sign of that happening. So given that (a) we have no idea what kind of a force could do this and (b) there is no sign of this alignment happening in practice - then Occam's razor says that we need not concern ourselves overly much with this issue. SteveBaker (talk) 12:33, 17 March 2010 (UTC)
- Valid point. I was not able to find any reputable source indicating that any alignment exists, even statistically, for all the galactic axes we have observed. If you think such an alignment does exist, you should find some evidence to support that belief; very likely, somebody else has studied this problem, and if there were a conclusion to be drawn, it's probably published in a reputable scientific journal somewhere. Nimur (talk) 06:24, 17 March 2010 (UTC)
- I'm referring to the central perpendicular galactic axis alignment and not to the alignment of matter within the Galaxy with the central perpendicular galactic axis. Although many things look random in nature (and in the presence of dark matter and energy throughout the Universe) you can not tell for sure just by looking. Instead you have to measure the angles of all the central perpendicular axis and determine what points in the Universe their imaginary projections intersect in order to know for sure. 71.100.11.118 (talk) 06:13, 17 March 2010 (UTC)
- Some work in the 1970s seems to indicate that inside a galaxy, certain structures align parallel to the galactic plane - see The alignment of interstellar dust clouds and the differential z-field of the galaxy, for example. However, this is not the entire galaxy aligning with respect to something in the universe - the alignment of individual galaxies appears to be random, and their position appears to be in the form of "strands" or superclusters. Nimur (talk) 03:46, 17 March 2010 (UTC)
- It is unlikely that galaxy-sized objects have any significant net electromagnetic properties. To my knowledge, no evidence of a galaxy-sized magnetosphere has ever been observed - it is not even clear what evidence could possibly exist to establish or discredit the idea of a galaxy-scale magnetic field alignment. Galactic magnetic fields seems to be a nice overview of the debate; and here is an IOP article about radio measurements of magnetism in the outer galaxy. Nimur (talk) 02:03, 17 March 2010 (UTC)
Chart
[edit]What is the PM and AM?174.3.107.176 (talk) 01:42, 17 March 2010 (UTC)
- I presume these are the morning (AM) and evening (PM) - "opening" and "closing" prices on the commodities market...but I suppose there are other possibilities. SteveBaker (talk) 01:54, 17 March 2010 (UTC)
- (ec) That chart represents spot prices for platinum on the London Platinum and Palladium Market. The Market's website has a brief history, which explains that statistics are kept for morning (AM) and evening (PM) spot prices (for historical reasons, a twice-daily record is kept). "In 1973 the London Platinum Quotation was introduced. It was the forerunner of the fixings; a twice-daily indication of the market price for spot platinum, reported by some of the principal companies dealing in the metal."[2] If you're interested in the origin of "AM" and "PM" as used in conventional time-keeping, see 12-hour clock#Abbreviations - ante meridiem and post meridiem. Nimur (talk) 01:56, 17 March 2010 (UTC)
- That's "weird". I always thought prices were determined by the less-then-second.174.3.107.176 (talk) 03:20, 17 March 2010 (UTC)
- Even in a modern electronic trading exchange, prices are averaged over a time-window. This New York Stock Exchange article from 2009 suggests that their computers run on a 62 millisecond latency (and soon to "improve" to single-digit millisecond latency); the effects of other system latencies mean that the actual price may be stale by the time an electronic transaction executed... but since the exchange is committed, it must go through with the new 62-ms window price. Back in the "old days", prices were averaged out over even longer periods, but with essentially the same hazard - if the price changes after a commit but before an execute, the trade must go through with the new price. You can see why less frenetic markets (like pork futures or the palladium market) might aim for once- or twice-daily price updates. Nimur (talk) 03:39, 17 March 2010 (UTC)
- The bid and offer prices quoted by individual market makers during the trading day do change on a second-by-second basis, as each market maker adjusts their prices depending on the supply and demand that they see in the market and their current positions (long or short). The twice-daily fixings are an additional pricing mechanism in which an auction process is used to determine a benchmark price. The benchmark price is used as a reference price for settling precious metals swaps, options and other OTC derivative trades. This is explained in the Guide to the London Precious Metals Markets - see the section headed "Dealing and Products".
- Returning to the original question, the chart shows 17 pairs of data points and the timeline is 17 years, so each data point could represent the average of the AM or PM fixings over a 12-month period - but without more information on the origins of the chart, it is difficult to be certain. Gandalf61 (talk) 10:09, 17 March 2010 (UTC)
- The image page for the chart claims that it's the contributor's own work (User:Bloublou) - so you could just contact the person who created it and ask. Bloublou's talk page seems under-used but there is an "Email this user" button in the menu to the left. SteveBaker (talk) 12:19, 17 March 2010 (UTC)
Question on Sapphire and Blue Emrald.
[edit]This is for the geology/ earth sciences experts out there. To cut a long story short I want to wear a blue sapphire set in gold ( for sentimental reasons) as a ring. Im not here to debate my faith or the pros and cons of wearing precious stones in rings. When I approached a trusted jeweller, and asked for the best Blue Sapphire he offered me a 9 carat blue Emrald. Im not an expert in stones but not daft either, I was told specifically to buy a blue sapphire, preferably a natural blue- deep blue - which is not heat treated. I listed out my requirements and the jeweller says Blue Emralds and blue sapphires are one and the same - just the name is different. He says the stones are from Sri Lanka though I was advised that the best Blue Sapphires came from Kashmir, India. Is a blue Sapphire same as a Blue Emrald? Structure wise and characteristics wise... Any gemmology experts out there who can help me? —Preceding unsigned comment added by 213.130.123.12 (talk) 04:37, 17 March 2010 (UTC)
- Your jeweler is either a) Bullshitting you on purpose or b) completely incompetant. Sapphires are a variety of corundum which also includes gemstones such as ruby. Emeralds are a variety of beryl, which includes gemstones such as aquamarine. They are completely different chemically. So my advice is to find someone else ASAP, since this guy is either dishonest or incompetant. --Jayron32 04:45, 17 March 2010 (UTC)
- I have to agree, no one should ever tell you that an emerald and a sapphire are the same thing, even with my lay knowledge this jeweler is either trying to rip you off or is completely incompetent. I would find it hard to believe that even the most ignorant of people in this trade would not know the difference in the two. Beach drifter (talk) 05:10, 17 March 2010 (UTC)
- I won't be as hard on the jewler. He may be an excellent artist but clearly doesn't know enough about emeralds and saphires to be trusted on that score. So feel free to trust him to set a stone in a ring, but trust yourself and these strangers on the interwebs to identify stones. See also saphire and emerald... reading my own links and those above I note no mention of blue emerald in either emerald or beryl, ultramarine is pale blue, and maxixe is a deep blue beryl (which fades with exposure to sunlight and can be restored with irradiation with neutron, gamma or x-rays). Other pale forms of beryl can be made into maxixe through irradiation.
- To further confuse things, pale blue corundum (saphire) is also called "oriental aquamarine" which could have confused your jewler -- a pale saphire is called aquamarine - a blue emerald.
- I will be at least that hard on the jeweler. One goes to a jeweler as an expert in jewels and jewelry, if he gets this wrong, he may later tell you that silver, gold, and platinum are just different forms of the same thing, or that 10 dollar bills and 50 dollar bills are the same thing. Good grief. Ralphcook (talk) 22:38, 20 March 2010 (UTC)
- So I don't think you'll have much trouble finding blue emerald (but called maxixe or aquamarine) but you'll have no chance of knowing whether it was dug from the ground blue, white or gold in colour, and note that x-raying isn't heat treating. --Polysylabic Pseudonym (talk) 07:55, 17 March 2010 (UTC)
Thanks for the answers. I for sure knew Sapphire isnt an Emrald but I thought technically a blue Sapphire was indeed a blue emrald. Thanks for the scinetific explanations, yes I have since done extensive online searches and I am going in for a 5 carat ( non heat treated ) blue sapphire. Thanks again. —Preceding unsigned comment added by 213.130.123.30 (talk) 08:54, 17 March 2010 (UTC)
My telescope
[edit]If my new telescope (the one with nearly a billion cameras all pointing at the same infinite point in space) finds a distant Galaxy that is 20 billion light years away then isn't the Universe over 15 billion years old or is there something weird going on here? 71.100.11.118 (talk) 06:00, 17 March 2010 (UTC)
- Yes, something weird is going on here. See Distance measures (cosmology). Basically, there are different ways of measuring distance on these large scales, and it is indeed possible to come up with a value in light years larger than the age of the universe. I welcome someone else to explain some of these different distance measures to me, because I sure don't understand them. Buddy431 (talk) 06:09, 17 March 2010 (UTC)
- An important question arises: how did you determine that the galaxy you (hypothetically) observed was 20 billion light years away? Did you use the redshift and Hubble's law? It's worth knowing some of the assumptions you are making. Nimur (talk) 06:29, 17 March 2010 (UTC)
- See Inflation for how the universe got to be bigger than its age in light years; and
- for the rest of the story see Observable universe which tells you in its lead section:
- The age of the Universe is about 13.7 billion years, but due to the expansion of space we are now observing objects that are now considerably farther away than a static 13.7 billion light-years distance. The edge of the observable universe is now located about 46.5 billion light-years away.
- --Polysylabic Pseudonym (talk) 08:12, 17 March 2010 (UTC)
The OP's proposal for an exhorbitantly expensive and complex array of CCD cameras that would somehow by means of vaguely described software match the performance of conventional reflecting or refracting telescopes was discussed last November. I conclude that the construction is not worth pursuing and it is never going to discover a new galaxy because it will not be built so the OP will never own one. Aside from the obsession about a "billion cameras", the OP's question is logical Cuddlyable3 (talk) 12:53, 17 March 2010 (UTC)
- Actually, the principle is already in use and quite simple and by using full fledged CCD camara with telescopic lens focused at infinity and not just CCD sensors the software merely combines the images coming from each camera to overcome the anomalies associated with each camera and its location. The principle of magnification beyond the individual camera lenses is in effect identical to that of any lens, refractive or reflective, in that there is more light in less space. The technique is clearly superior to lenses, however, in that the distortion from lens curvature is eliminated. Think about it. Also I'm not obsessed with it anymore than I am with the purchase of my next electric vehicle. 71.100.11.118 (talk) 10:02, 19 March 2010 (UTC)
- Remember that seeing a galaxy 13.7 billion light-years away means you're looking 13.7 billion years back in time. So the galaxy may not be there any longer. ~AH1(TCU) 16:26, 17 March 2010 (UTC)
- Is that equivalent to saying that the galaxy is still where it was but you are no longer where you were? Cuddlyable3 (talk) 17:22, 17 March 2010 (UTC)
- No, not at all. --Mr.98 (talk) 23:29, 18 March 2010 (UTC)
- Is that equivalent to saying that the galaxy is still where it was but you are no longer where you were? Cuddlyable3 (talk) 17:22, 17 March 2010 (UTC)
Decomposition
[edit]Assuming an average of all conditions, how long would it take for an unembalmed human corpse to completely decompose (bones and all) after being buried 6 feet underground with shrouds but no casket? My goal is to have a time frame for which, theoretically speaking, one could plow over and/or dig for construction purposes without having any indication that the land had been used as a graveyard. DRosenbach (Talk | Contribs) 13:39, 17 March 2010 (UTC)
- I'd imagine there are too many factors to work out a reliable time frame. Temperature, oxygen availability, scavengers, humidity etc. are all factors in the speed of decomposition, so without a specific location I doubt it'd be possible to work out as a general rule of thumb. Sorry to be so unhelpful. Regards, --—Cyclonenim | Chat 14:27, 17 March 2010 (UTC)
- Agreed, but it's likely to be on the order of thousands of years for bones and teeth to completely decompose. Also note that human bodies are likely to be buried with items which don't decompose, such as gold jewelry or stone tools. StuRat (talk) 14:30, 17 March 2010 (UTC)
- If the conditions are favorable the bones might never decompose and start fossilizing instead. Under extremely favorable conditions even the soft parts might fossilize. Dauto (talk) 15:23, 17 March 2010 (UTC)
- It's really so variable? That's surprising! OK...how about Jerusalem and how about the NY Metro area. DRosenbach (Talk | Contribs) 15:29, 17 March 2010 (UTC)
- The specific information you are requesting may not be in our article on Body farm, but it may be a good place to start. 10draftsdeep (talk) 15:49, 17 March 2010 (UTC)
- It's really so variable? That's surprising! OK...how about Jerusalem and how about the NY Metro area. DRosenbach (Talk | Contribs) 15:29, 17 March 2010 (UTC)
- If the conditions are favorable the bones might never decompose and start fossilizing instead. Under extremely favorable conditions even the soft parts might fossilize. Dauto (talk) 15:23, 17 March 2010 (UTC)
Wait a while longer before you plough over this gentleman. Cuddlyable3 (talk) 17:19, 17 March 2010 (UTC)
Digoxin in health
[edit]From the digoxin article, cardiac glycosides primarily have negative chronotropic/positive ionotropic effects on the heart. While such effects are deemed therapeutic for patients suffering from forms of heart failure, etc., how are such effects so harmful as to be toxic to humans who do not exhibit heart failure. The focus of my question is on consumption of milkweed (or foxglove for that matter) -- is the toxicity of the plant related to the high concentrations of the glycoside? Because if it's not, I don't see how the cardiac effects I indicated above would be all that harmful. DRosenbach (Talk | Contribs) 13:45, 17 March 2010 (UTC)
- Why would cardiac glycosides have a negative chronotropic effect? Chronotropics affect the heart rate, and cardiac glycosides cause tachycardia in high doses. I don't know how this relates specifically to your question so I'll let someone answer more directly, but cardiac glycosides (such as those present in foxglove) do have the ability to cause tachycardia and eventually fibrillation. Regards, --—Cyclonenim | Chat 14:23, 17 March 2010 (UTC)
- Digoxin blocks the atrioventricular node and causes bradycardia — i.e. negatively chronotropic. Digoxin is useful in some heart conditions where the heart rate is too fast (tachyarrhythmias). Digoxin toxicity includes heart block. Axl ¤ [Talk] 08:39, 19 March 2010 (UTC)
Animal clitorises
[edit]Do most mammals have them, and if so, are they external, or is the external clitoris exclusive to humans (or primates maybe)? I hear tell that pigs have them internal to the vagina. External raises certain evolutionary questions, as you can imagine. How come masturbation isn't selected against since it would seem to distract from reproduction? Is it the vague benefit of comfort, or some social function? 86.21.204.137 (talk) 15:08, 17 March 2010 (UTC)
- Clitoris certainly implies, but does not actually state, that most mammals have them. Lfh (talk) 16:03, 17 March 2010 (UTC)
- It is rather difficult to select against. If sexual intercourse is pleasurable, then anything that simulates sexual intercourse will be pleasurable. It does seem to be selected against to some extent, though - actual sex is generally more pleasurable than masturbation (this isn't just anecdotal, there have been studies that have shown the relevant hormones stay in the body longer, or something, after sex than after masturbating). --Tango (talk) 16:25, 17 March 2010 (UTC)
- [citation needed]. Please cite references. I believe Kinsey (and Masters and Johnson as well) contradict you on the claim that masturbation is less pleasurable than intercourse, at least for the perceived strength of orgasm. Comet Tuttle (talk) 17:28, 17 March 2010 (UTC)
- Furthermore, since a clitoris is basically a homolog to the penis glans, it would seem to be somewhat evolutionarily impossible to see a species where the male had a penis, but the female has absolutely no clitoris. Males of all mammals have nipples, and the same number and distribution thereof as females, even if they don't give milk, sexual dimorphism in mammals can give rise to variations between the sexes, but you don't have any situations where entire organs in one sex are completely missing from the other. You have modifications to the same set of organs, but you can recognize homologous structures between both sexes. Now, whether a female of a species has a functional clitoris in the sense that humans do may be a different question (i.e. does the female's clitoris in say, a badger, provide the same level of pleasure as it does in humans) or if such structures in other species are entirely vestigal is another question. But I suspect you will find the structure itself, or one very like it, in all females of species where the male has a penis. --Jayron32 17:24, 17 March 2010 (UTC)
- Our masturbation article has a lot of material about the original poster's latter questions, including an "Evolutionary utility" section and a "Benefits" section. Comet Tuttle (talk) 17:28, 17 March 2010 (UTC)
- Gross. —Preceding unsigned comment added by 99.254.8.208 (talk) 05:19, 18 March 2010 (UTC)
Time Dilation
[edit]Does Gravity slows Time or makes it Fast? —Preceding unsigned comment added by 59.161.88.15 (talk) 17:07, 17 March 2010 (UTC)
- A distant observer watching a clock that is near a massive object will see it ticking more slowly than a clock held by the observer. --Tango (talk) 17:09, 17 March 2010 (UTC)
- So yes, gravity slows time down. --The High Fin Sperm Whale 17:19, 17 March 2010 (UTC)
- Gravitational time dilation has a slightly less cryptic version of what Tango said: "the lower the gravitational potential (closer to the center of a massive object), the more slowly clocks run". Zain Ebrahim (talk) 19:40, 17 March 2010 (UTC)
- ***WP:OR alert *** Exception: The gravity of an urgent deadline will make time go faster, asymptotically faster the nearer one gets to it.[citation needed] Clarityfiend (talk) 20:09, 17 March 2010 (UTC)
So how fast does time go if you are alone in the universe (and exert no gravity on yourself)? —Preceding unsigned comment added by 99.254.8.208 (talk) 05:18, 18 March 2010 (UTC)
- Exactly one hour per hour (but it will seem longer).--Shantavira|feed me 08:24, 18 March 2010 (UTC)
- That is why I gave a "cryptic" answer - the simpler answer quoted by Zain is misleading. It is important to keep in mind that time dilation is a relative thing. It makes no sense to ask how fast one clock runs - it will run at one second per second. You have to compare how two clocks run. There is no "absolute time" to compare a solitary clock to. --Tango (talk) 08:57, 18 March 2010 (UTC)
nitrogen temp
[edit]i have a bottle of nitrogen (nitro) for paintball and it hot if its full and cold when its near empty. why is this? once its released will it still be cold or hot or does it become room temp very fast? —Preceding unsigned comment added by Thekiller35789 (talk • contribs) 18:02, 17 March 2010 (UTC)
- The ideal gas law (N2 can be considered an ideal gas) states that PV=nRT. V, your volume is constant, as are the variables n and R, so P and T have a linear relationship. Thus the higher P (pressure), the higher T (temperature) and v.v. Googlemeister (talk) 18:09, 17 March 2010 (UTC)
- (ec) Per the ideal gas law, pressure and temperature are directly proportional to each other. In this case, as pressure drops (as you expend the nitrogen cartridge), the temperature of the gas drops, too. This then cools the cartridge itself. If you let the cartridge sit (either pressurized or unpressurized) it will move towards room temperature. — Lomn 18:12, 17 March 2010 (UTC)
- Another way to think about this is that as the gas expands and does work (accelerating the paintball and pushing against the atmosphere), the gas loses thermal energy, and is thus colder. It will become room temperature eventually, (probably within hours, depending on the size and material of the bottle). Buddy431 (talk) 20:18, 17 March 2010 (UTC)
- See adiabatic cooling. --Jayron32 20:22, 17 March 2010 (UTC)
- Thanks, Jayron. I knew there was a more specific term than what I used, but it wouldn't come to mind. Now that mystery is solved! — Lomn 01:34, 18 March 2010 (UTC)
i have has this bottle for years and it does not change temp. is always hot or cold. —Preceding unsigned comment added by Thekiller35789 (talk • contribs) 21:26, 17 March 2010 (UTC)
- That would violate the laws of thermodynamics. — Lomn 01:34, 18 March 2010 (UTC)
- Not if it was always in a hot or cold environment :) Buddy431 (talk) 02:03, 18 March 2010 (UTC)
if you eat too much, will your body not digest it?
[edit]If you eat too much, so that you are stuffed, will your body not digest some of the extra food as normal? Also, insofar as it wouldn't, is the same true for someone who is "stuffed" because their stomach has shrunk due to their consistently eating very, very little, but after a long time of that routine, eating a (to them) very large meal? Thanks. 82.113.121.167 (talk) 18:22, 17 March 2010 (UTC)
- Well, if you eat too much you will make yourself vomit and that food won't get digested. Other than that, I don't know of any reduced efficiency of digestion following excessive consumption. --Tango (talk) 18:28, 17 March 2010 (UTC)
- That's interesting, it's not what I'd supposed. I'm not trying to quibble here, I'm not "asking for a reference" but it sounds like you have a better idea of what search terms to use than I would. Could you tell me how to use Google, what keywords and so on, to find something more formal to this effect? Thank you. 82.113.121.167 (talk) 18:44, 17 March 2010 (UTC)
- I'm guessing what the OP should ask is if the body would run out of enzymes or stomach acid needed for digestion. I believe it just takes longer to digest and the body would keep on producing the necessary fluids. Digestion also takes a lot of energy, but I don't think the body would stop digesting because it's running low on energy. And yes, providing you can keep the food down. There IS a limit of how much you can stuff yourself based on stomach volume and how high the food can pile up in the esophagus. --Kvasir (talk) 18:46, 17 March 2010 (UTC)
- My only thought is that a great amount of food will take longer to digest. If it truly went undigested then it wouldn't come out the other end in a radically different format. Vranak (talk) 19:25, 17 March 2010 (UTC)
- If you eat a very large amount of vegetables, then it comes out quite green at the other end. Eating lots of vegetables is a good way to feel full without eating too many calories. 92.29.150.112 (talk) 19:58, 17 March 2010 (UTC)
- I do think that the food will be less fully digested if you eat more than your digestive system can handle. For one thing, a few hours after you eat, your stomach contents will drain into the small intestine, pushing everything else along, whether it's been fully digested or not. For an extreme example, those who win international food eating contests typically lose the extra food in short order, out one end and/or the other. I think digestion efficiency also varies by person and by age, as some people can eat as much as they want without gaining weight, while others seem to gain weight if they eat even a little extra. StuRat (talk) 23:23, 17 March 2010 (UTC)
- I can find reference to large calorific intake in two situations: competitive bike riding (see [3]) and Antarctic exploration (see [4]). Both situations use about 6000 calories per day. I can't find my reference, but I remember reading Lance Armstrong's book and think he said that the body struggles to digest more than about 6000 calories per day. --Phil Holmes (talk) 12:01, 18 March 2010 (UTC)
- Yes, and I'd expect athletes to be more efficient at digestion, in general, too, since, if they weren't, they would carry extra weight in undigested food and/or have less energy, both of which would put them at a competitive disadvantage. StuRat (talk) 14:24, 18 March 2010 (UTC)
- Any refs Stu? Or is that just your gut feeling?--79.76.137.66 (talk) 00:30, 19 March 2010 (UTC)
- Neither, it's a logical conclusion. StuRat (talk) 03:35, 19 March 2010 (UTC)
- Not sure I agree with that. The reason athletes can eat so much and not put on excess weight is because they use it up the energy stored during exercise, not because they're "more efficient at digestion". The only way digestion could be more efficient is if you're digesting stuff quicker, but even so you'd still end up with the same amount of end product in your body. The only way not to put on the excess weight is to use the energy during exercise, which athletes do copiously. Regards, --—Cyclonenim | Chat 14:39, 19 March 2010 (UTC)
- The speed of digestion isn't what I'm talking about, but rather how many calories and nutrients are absorbed for a given bit of food, which is likely to be inversely related to the speed of digestion. StuRat (talk) 17:54, 19 March 2010 (UTC)
Cyclooxygenase-1 in thrombocytes
[edit]How much cyclooxygenase-1 does a single thrombocyte contain? Thanks in advance for the answers! Icek (talk) 19:02, 17 March 2010 (UTC)
Organic chemistry
[edit]What are the steps for making an anti-septic using parachlorometaxylenol,castor oil soap,pine oil,caramel,isopropanol and water. —Preceding unsigned comment added by 80.87.82.1 (talk) 19:04, 17 March 2010 (UTC)
- "Mix" is the major one. Parachlorometaxylenol is the active ingredient, the others are the vehicle for making it into an easy-to-handle form with reasonable spreadability and concentration, minimal irritation or other side-effects, and enhanced activity. DMacks (talk) 19:22, 17 March 2010 (UTC)
- Nothing posted here constitutes medical advice, or constitutes advice as to its safety or effectiveness. See [5] which is a book "Vanity, vitality, and virility: the science behind the products you love to buy" (2004) By John Emsley, found at Google book search by searching for the ingredients listed. The parachlorometaxylenol was first produced in 1923 and is also known as Dettol, (under which name we have an article) or PCMX, manufactured by Reckitt Benckiser. The article says it is sold as a 5% solution of PCMX with 5% pine oil, 14% castor oil soap, and 12% isopropanol, with the last 3 ingredients helping to keep the PCMX in solution. The ingredients listed only add up to 36%, so something else must be present. The manufacturer's data sheet for Dettol lists Chloroxylenol 4.8%. That seems to be a new name for the older PCMX, as a manufacturer's website suggests, and PCMX redirects to the article Chloroxylenol. (I've never understood why chemists give the same compound multiple chemical names. Is it like adding (then removing) tailfins to cars, or making neckties wider then narrower, or changing hemlines on dresses? Change for the sake of change, or new understandings of the molecular structure?). Edison (talk) 19:44, 17 March 2010 (UTC)
choking
[edit]is it possible that some people are immune to bloodchokes> i.e. (rnc) ? i take jujitsu and i have asked others to try to choke me unconscious and they were unable to do so. some have also used ropes. obviously eventually i would die because it also a oxygen choke (closes windpipe). but that takes over 1 minute. bloodchokes take no longer than 10 secs. they applied it for 40 secs. it was done by other jujitsu students —Preceding unsigned comment added by Thekiller35789 (talk • contribs) 22:00, 17 March 2010 (UTC)
- I think this question might fall under a medical advice guideline; something to the effect of "consult a physician before beginning a rigorous martial arts training regimen where you might be subject to people choking you." Nimur (talk) 22:03, 17 March 2010 (UTC)
- OP - do not ask your fellow Jujitsu students to choke you. Nobody, including yourself, is immune to hypoxia caused by a chokehold; if the hold is done correctly, it can seriously harm you. Nimur (talk) 22:04, 17 March 2010 (UTC)
lol, this is not medical advice. —Preceding unsigned comment added by Thekiller35789 (talk • contribs) 22:11, 17 March 2010 (UTC)
btw i saw an asphyxia vid of a woman doing a full suspension hanging on herself off a chair so it is possible she lasted 20 secs until her husband let her down she did not pass out thou you could HEAR her choking horribly so he let her down. —Preceding unsigned comment added by Thekiller35789 (talk • contribs) 22:14, 17 March 2010 (UTC)
- The reference desk is not a forum. Your original question, "is it possible that some people are immune to bloodchokes", has been answered: it is not possible. If you have additional science questions, that is fine. If you would like to discuss asphyxiation in general, this is not the appropriate venue. Nimur (talk) 22:17, 17 March 2010 (UTC)
i dont wish to discuss "asphyxiation in general" my question was not answered. simply stating "no" is not an answer. how do you explain what iv stated ? —Preceding unsigned comment added by Thekiller35789 (talk • contribs) 22:28, 17 March 2010 (UTC)
- "No" is an answer. Maybe the people trying to choke you out were doing it wrong or they did it wrong on purpose because they realize how utterly moronic it is to ask someone else to choke you into unconsciousness. 22:41, 17 March 2010 (UTC)Burpelson AFB (talk)
- Unless you've somehow managed to find an alternate way to oxygenate your brain, then no, no you are not immune to lack of bloodflow to your head. They did it wrong. --Mask? 22:44, 17 March 2010 (UTC)
they were black belts they didnt "do it wrong" —Preceding unsigned comment added by Thekiller35789 (talk • contribs) 22:47, 17 March 2010 (UTC)
- people have variations in the musculature and other structures of the neck, as well as differences in the circulatory system more generally (due to physical development or genetics) that can impact on their susceptibility to this. all this act involves is blocking off blood flow through the carotid artery to a sufficient extent to starve the brain of oxygen, inducing a faint. if the person doing it fails to block off a sufficient amount of blood (because the artery isn't where expected, or is protected by muscles, or has a higher than normal amount of blood pressure, or...), the effect won't happen. Someone expert in the technique can likely overcome any such problems to make it work regardless.
- that being said, you're an idiot. even sub-feinting episodes of oxygen deprivation can cause an amount of brain damage. further, your fixation on this issue is problematic - I am thoroughly surprised that your sensei hasn't beaten your ass but good over this, and I disrespect him because of it. I suggest that you tell him immediately that you have been practicing potentially lethal techniques without cause or supervision, and if he doesn't have you scrubbing the dojo for the next six months I suggest that you drop him and find a teacher who is capable of teaching you proper discipline. --Ludwigs2 22:49, 17 March 2010 (UTC)
- Who cares if they were "black belts"? Even Pat E. Johnson does something wrong from time to time. You've had your answer, if you don't like the free answers, ask for a refund. Or, if you would rather hear "Yes, you're so manly that you can't be choked out" I'm willing to accept $10. You could even quote me. Burpelson AFB (talk) 22:58, 17 March 2010 (UTC)
- They did, in fact, 'do it wrong'. If the desired result is to block off oxygen to your brain and make you pass out, which you did not do, then they, by definition, did it wrong. You dont have metal blood vessels. --Mask? 22:57, 17 March 2010 (UTC)
for one thing my teacher is fine with it. we do it in class to each other occasionally. secondly the vid i mentioned which i can link if you want proves this is possible. im looking at this from a scientific viewpoint. if a rope is choking someone and their dangling in the air thats about as tight as it going to get. —Preceding unsigned comment added by Thekiller35789 (talk • contribs) 23:25, 17 March 2010 (UTC)
- then your teacher is an idiot to. your behavior shames him, shames your school, and shames you. with that, I'm closing this question. --Ludwigs2 23:29, 17 March 2010 (UTC)
you dont have the right to close this, no one made you god. im honestly looking for a scientific explanation for this. you dont have any right to insult me. no one answered me about the hanging question.—Preceding unsigned comment added by User:Thekiller35789 (talk • contribs)
- Like all harmful things, there is a spectrum of tolerance. Many factors, including exposure time, method of application of the hold, etc., will all affect how soon a person faints, suffers brain damage, and eventually dies. Because of the fatal nature of this subject, it is probably impossible to get an LD50 for choking time for humans. Here is a study on Inter and intraspecies genetic differences in survial to an acute hypoxic challenge in mice, rats, quails and chickens. As you can see, there is a scientific answer: this activity will result in brain damage and death. It is hard to say exactly how much force, or how many seconds, are required - for you, or for any "average individual." There will be outliers on each end of the spectrum, who survive longer or die sooner, due to many factors. For this reason, you should avoid participating in such an activity, because there is a real risk of permanent brain damage and death. If your martial arts teacher approves of such lethal play, he is not a very good instructor. Nimur (talk) 00:28, 18 March 2010 (UTC)
well generally it takes 5-10 secs to go to sleep, if the hold is held after 3 minutes their will be brain damage and if it is still held after ten minutes there can be death. it is safe as long as they let go after you pass out. but im not hear to talk about safety. what i want to know is how that woman was still conscious after 20 secs of FULL suspension hanging. —Preceding unsigned comment added by Thekiller35789 (talk •
contribs) 00:51, 18 March 2010 (UTC)
- That's nonsense. Victims do not "go to sleep", they fall unconscious. Whenever you lose consciousness, there is a significant risk of permanent brain damage. Nimur and Ludwigs are right. Your teacher is an idiot. "That women" probably did not have her carotid artery closed, but only choked. It's fairly easy if not always pleasant to do without breathing for 20 seconds. --Stephan Schulz (talk) 01:00, 18 March 2010 (UTC)
- In judo class I was choked into unconsciousness numerous times, And it did not affect me. me. me. Edison (talk) 03:44, 18 March 2010 (UTC)
how do you not have your carotid artery closed from FULL suspension hanging. thats as tight as it gets
- Well, it depends on a couple factors, including the weight of the person, and the physiology of their circulatory system in their neck. You'd probably be interested in our article Hanging, and see this page on hanging for more information on suspension strangulation. According to that, it's normal to struggle for 1-3 minutes, if the person is hanged without a drop. Indeterminate (talk) 03:11, 18 March 2010 (UTC)
yes, i already read those the second link you gave is not a reliable source as it states "the prisoner died "almost without a struggle” and they would be seen to writhe in pain for just a few seconds, if at all, before going limp." in the same paragraph. getting chocked out is not supposed to take 1-3 min. as seen here http://www.youtube.com/watch?v=SnkSxIMGTPU&feature=related —Preceding unsigned comment added by Thekiller35789 (talk • contribs) 04:59, 18 March 2010 (UTC)
i should also note it only takes 10 pounds of pressure the close the arteries in the neck. (the same to break a egg) —Preceding unsigned comment added by Thekiller35789 (talk • contribs) 05:25, 18 March 2010 (UTC)
- Why should you note that? This is a reference desk, not a forum for frankly mastubatory postings about choking. You appear completely unwilling to accept any answer that has been given to you. I suggest whatever purpose this question had has now been served. --Tagishsimon (talk) 05:29, 18 March 2010 (UTC)
- Maybe they are purposely doing it wrong in order to avoid murder charges. 67.243.7.245 (talk) 15:29, 18 March 2010 (UTC)
no they wernt " purposely doing it wrong" they did it right and very hard too. i jard red marks on my neck for 2 days afterwards. and too Tagishsimon i "noted" it because if it only takes 10 pounds i dont see why that woman could still be conscious. —Preceding unsigned comment added by Thekiller35789 (talk • contribs) 16:18, 18 March 2010 (UTC)
- I haven't watched the video in question, nor do I want to. But clearly you are missing the obvious facts which we have stated numerous times above. There are lots of ways to be harmed by such activities. Choking, asphyxiation, hypoxia, strangulation, breaking of the neck, and so on, can all occur; and can all result in permanent harm. Sometimes, these do not occur, but it's hard to say exactly what threshold is necessary. How are you having such a hard time understanding this simple concept? It depends on how the activity was conducted - a specific numerical measurement of the necessary force is hard to provide, especially since controlled experiment has not really been conducted on "how to kill by strangulation". If the video shows somebody who did survive, then by tautology they were not subject to enough force to kill them. If you would like other examples where this is not the case and the subject died, you can search for snuff films, but these are probably illegal in the part of the country where you live. Your continued stubborn behavior and your infatuation with death and strangulation has raised concern, and currently a debate is taking place on the Administrator's Notice Board about the best way to deal with your behavior, which is approaching the limits of what is permissible on the Reference Desk and elsewhere in Wikipedia. Nimur (talk) 16:35, 18 March 2010 (UTC)
- What are you really asking here? Are you asking if you're a super-human who can survive even with his cardioid arteries pinched shut? I think it's safe to say that the answer to that question is "no". APL (talk) 00:52, 20 March 2010 (UTC)
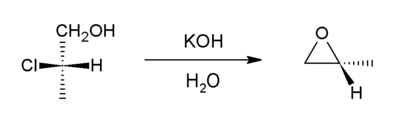
don't we want to use non-nucleophilic base to form epoxides? (unwanted diols)
[edit]Why do our articles mention potassium hydroxide as a base catalyst when halohydrins or epoxides are attacked? Isn't t-butyl hydroxide a better choice? The hydroxide also seems quite likely to react with the halide on the halohydrin (forming a diol). It seems that even when you do form the epoxide, it would get hydrolysed to form a diol. John Riemann Soong (talk) 22:03, 17 March 2010 (UTC)
- t-butyl hydroxide is tert-Butanol, which isn't much of a base at all, being an alcohol, it will not have much of an affect on base-catalyzed reaction. Organo-metallic (Grignard) t-butyl reagents are very strong bases, like tert-Butyllithium, but these are so strong that they will actually do a very different set of reactions. If you are looking for a hydroxide-like base which is stronger than potassium hydroxide, then perhaps something like sodium amide may be better. --Jayron32 01:41, 18 March 2010 (UTC)
See Non-nucleophilic base for some options. You don't want any kind of hydroxide, because OH− is the nucleophile causing trouble. This is assuming your guess is right, and you don't end up with much of your desired product — maybe the reaction does work fine with KOH, in which case your question will change to "why doesn't it cause problems?".
Give us a concrete example (or a link) and we can discuss it.
Ben (talk) 04:31, 18 March 2010 (UTC)
- Err, I meant t-butyl O- (I guess I meant to say tert-butyl alkoxide). I was looking at the synthesis of epoxides and also epoxide#reactions. Why is a nucleophilic base okay here? John Riemann Soong (talk) 05:12, 18 March 2010 (UTC)
- AAAAHHH! Tert-butoxide is a different story. In that case, yes, tert-butoxide is very good base, it prefers the E2 elimination mechanism over substitution (see Elimination reaction and Nucleophilic substitution) due to its high pKa. See Potassium tert-butoxide for some applications. So that may solve your diol problem. --Jayron32 05:30, 18 March 2010 (UTC)
- Yeah but I guess my question is -- why aren't you required to use a non-nucleophilic base? (I haven't worked with epoxides in the lab yet so I have no practical experience in this area).
- Also side question -- why is potassium tert-butoxide more popular than sodium tert-butoxide? Isn't the sodium species cheaper? John Riemann Soong (talk) 05:38, 18 March 2010 (UTC)
- Regarding the mechanics of epoxide opening; no idea. Ask one of your professors about it. Regarding the sodium vs. potassium choice; it probably has to do with subtle differences between the counterions. Sodium is a harder (smaller, denser) ion than potassium, so the softer potassium ion may have some effects which make it more useful to have in the reaction. See HSAB theory for a discussion of chemical hardness pertaining to Lewis acids and bases like sodium and potassium cations. --Jayron32 06:38, 18 March 2010 (UTC)
K salts are often perferred to Na salts where the sodium version is hygroscopic and this is undesirable (especially in analytical work, where want a fixed composition for weighing). Examples I could find on WP are:
- Potassium_hydroxide#Niche_applications: "In chemical synthesis, the selection of KOH vs. NaOH is guided by the solubility for the resulting salt."
- Potassium permanganate is much more commonly used than sodium permanganate
- Potassium hydrogen phthalate is used as a primary standard in acid-base titrations; sodium hydrogen phthalate doesn't have an article!
- Potassium chlorate is not hygroscopic, unlike sodium chlorate
- Potassium nitrate is preferred to sodium nitrate in gunpowder because "The major problem of using the cheaper sodium nitrate in gunpowder is its tendency to go damp."
- I couldn't find anything on potassium cyanide vs. sodium cyanide, but I'm willing to bet hygroscopy is an issue for some uses of NaCN, so KCN is used
Ben (talk) 14:32, 18 March 2010 (UTC)
- The only mention of KOH at Epoxide is Epoxide#Intramolecular_SN2_substitution, so that's what I've thought about.
- Here's the mechanism I'd expect:
- and the unwanted side-reaction I think you're wondering about is this:
- Having a picture of it in front of me made it much easier to think about (there's a lesson here!). The desired intramolecular reaction (ring-closing SN2) should easily dominate over the undesired intermolecular reaction (simple B-replacing-Cl SN2), because intramolecular reactions are usually about 103 times faster (so I was told in first year).
- I imagine that it's easier for a base to deprotonate the OH than to do SN2 on the Cl, because (1) deprotonation is less sterically hindered in this case, and (2) the OH probably already has some sort of interaction with the Cl, with an oxygen lone pair donating into the C-Cl σ* antibonding orbital to stabilise the molecule.
- Am I right?
- I think you are generally right here; however the 1000x faster for intramolecular condensations vs. intermolecular condensations is mitigated somewhat here by the ring strain of the three-membered epoxide ring. Ring closure is optimized for 5- and 6- membered ring products; smaller rings suffer from ring strain, while longer chains suffer from entropic effects (the ends can't "find" each other). --Jayron32 16:59, 18 March 2010 (UTC)
- Excellent point.
- John, if you read the reference for this reaction, you'll see that the yield was 67%, so it obviously works pretty well, whatever the reason!
- Yeah that makes me think. Thanks so much for your diagrams, Ben. I can accept that deprotonation (of an alcohol) is generally faster than SN2 ... and maybe the chloro group makes the alcohol more acidic (by inductance and hyperconjugation). But the epoxide product itself is vulnerable to ring opening, isn't it? The issue is that I can see a forward reaction from the epoxide to a diol, but it's comparatively harder to get from the diol to the epoxide under basic conditions. Does the yield decrease if the reaction is run for too long? John Riemann Soong (talk) 23:43, 18 March 2010 (UTC)
I reckon the epoxide doesn't suffer much ring-opening because it's not very soluble in water, so it floats on top of the aqueous layer which contains KOH.
- Apparently, propylene oxide is appreciably soluble in water.
- Well in the same way that "ether" is kind of appreciably soluble, right? What does appreciable mean? John Riemann Soong (talk) 21:03, 20 March 2010 (UTC)
Google says 40.5 wt.% @ 20C - according to JTBaker and Dow.
Ben (talk) 13:18, 21 March 2010 (UTC)
- Interesting... I mean, at first glance propylene oxide doesn't seem very different from diethyl ether in terms of solubility. I wonder if the banana bonds make the C-H bonds polar enough, especially with the electronegative epoxy oxygen, along with the H-bonding being less hindered... John Riemann Soong (talk) 21:44, 21 March 2010 (UTC)
New York State Regions
[edit]Which regions of New York show evidence that crustal uplift was dominant over erosional forces in the past? Please help me answer this question.--Lamb99 (talk) 23:47, 17 March 2010 (UTC)
- I came from a region in New York where the erosion from the last glaciation created great gorges and scars, the Finger Lakes and Watkins Glen, among others. Here's a hint: look at a topographic map of New York State (like this one), and find places where you do not see such glacial erosion. Crustal uplift is part and partial to orogeny - mountain building - so look for parts of the Allegheny Plateau, Catskill Mountains, and Adirondacks which are less eroded than others. In addition to glaciation, there are a few key rivers in New York State - the Susquehanna River and Hudson River. The effects of water course erosion on a drainage basin are dramatically different than a glacial receding scar. Nimur (talk) 00:15, 18 March 2010 (UTC)
- Isn't there also crustal uplift from the weight of a glacier being removed, to maintain isostatic equilibrium? —Preceding unsigned comment added by 66.133.196.152 (talk) 04:35, 18 March 2010 (UTC)