Wikipedia:School and university projects/MetRaZymes University of Stuttgart


This workshop will take place on June 6th and 7th, 2024 in collaboration between Wikimedia DE (or WMDE), German chapter of Wikimedia, and doctoral students of the program Network “Metal-Containing Radical Enzymes (MetRaZymes)” funded as Marie Skłodowska-Curie action. The training will be carried out by RudolfSimon, and Ideophagous.
The location is in Stuttgart, Universitätsstrasse 32 (Eingang über Universitätsstr. 34), Room U32.119
The Network MetRaZymes brings together chemists, physicists and biologists for research and training in the field of enzymes and proteins that contain metal ions.[1] This research area is not yet well-covered in Wikipedia, but walks in the steps of other, similar programs around the world. During this two-day workshop, the students will learn how to create and edit relevant Wikipedia entries. This will then be followed by assignments (in collaboration with their Principal investigators) in which the students will have to edit Wikipedia to improve content and/or to create entirely new articles.
Outline
[edit]The outline of the workshop is as follows:
Thursday, June 6th
[edit]
- 09.15 - 12.30: Introduction to Wikipedia (Room U32.119)
- Understanding Wikipedia and its ecosystem, how to write an article, relevance, structure, references, templates, rules, style, toolbox, user account, monitoring, statistics, create a sample article.
- 12.30-13.30: Lunch break (Mensa)
- 13:30-16.30: Editing with tutors (Room U32.119)
- 16:30-17:30: Review of work in process, issues, questions (Room U32.119)
Friday, June 7th
[edit]
- 9.15-10.15 Introduction to Wikimedia Commons (Room U32.119)
- How to handle and use photos and graphics to illustrate an article
- 10:15-12.30: Editing with tutors (Room U32.119)
- 12.30-13.30: Lunch break
- 13.30-15.30: Editing with tutors (Room U32.119)
- 15.30-16.30: Participants present the articles they created and their learnings (Room U32.119)
How to edit Wikipedia
[edit]See: Editing Workshop for the general outline of the editing workshop.

Articles
[edit]- Anatomy of an article (example Protein), pay attention to these concepts:
- Title
- Status (good, protected)
- Intro
- Top picture or infobox
- Table of content
- Middle sections and sub sections
- Other inner elements (internal links, pictures, tables, formulas, notes, references)
- Bottom sections (Notes, References/Sources, External links, See also, Further reading)
- Navigational templates
- Categories
- Visual Editor vs Source Editor
- Overview of visual editor interface
- Start editing
- Wikipedia:Manual of Style
Talk page
[edit]- Anatomy of a talk page (empty, full)
- To crete a new discussion, click on "New Section"
- Follow the etiquette and regulations (see below)
- Tag the editor(s) you want to address (if any)
- Resolve disputes politely, and if it doesn't work, escalate to the next level as explained in the dispute resolution page (linked below)
- Wikipedia:Etiquette
- Help:Introduction to talk pages/1
- Wikipedia:Dispute resolution
The basics
[edit]Here is a link to tutorials that can help you edit Wikipedia: Wikipedia:Tutorial and Training Materials.
- Each assertion you type needs to be verifiable: Make sure that you add references to back up statements.
- Your references need to be secondary sources: original research is not accepted as a reference.
- Avoid plagiarism: This may include your own work, unless it is published under a free license allowing for commercial and noncommercial use. Plagiarism amounts to a breach of copyright, which violates Wikipedia policy. If you need to use work you have published under a copyright license, please, reformulate.
- Neutral point of view: Articles should be written from a neutral point of view. You should represent fairly, and as far as possible without bias, all significant views that have been published by reliable sources.
More useful links
[edit]- Wikipedia:Cheatsheet
- Wikipedia:Simplified ruleset
- Wikipedia:WikiProject_Biophysics
- Wikipedia:WikiProject_Biophysics/Assessment
Useful templates
[edit]To be put both directly and between nowiki brackets in order to show the code.
Infoboxes
[edit]Some articles require infoboxes. Infoboxes always appear on the right hand side of articles, and have to be placed before the rest of the article's text.
Three examples
[edit]This is an example of the chembox (chemical compound infobox) for methane.
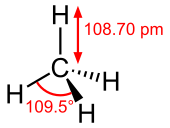
| |||
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Methane[2] | |||
| Systematic IUPAC name
Carbane (never recommended[2]) | |||
Other names
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| 3DMet | |||
| 1718732 | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| EC Number |
| ||
| 59 | |||
| KEGG | |||
| MeSH | Methane | ||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII | |||
| UN number | 1971 | ||
| |||
| |||
| Properties | |||
| CH4 | |||
| Molar mass | 16.043 g·mol−1 | ||
| Appearance | Colorless gas | ||
| Odor | Odorless | ||
| Density | |||
| Melting point | −182.456 °C (−296.421 °F; 90.694 K)[4] | ||
| Boiling point | −161.5 °C (−258.7 °F; 111.6 K)[4] | ||
| Critical point (T, P) | 190.56 K (−82.59 °C; −116.66 °F), 4.5992 MPa (45.391 atm) | ||
| 22.7 mg/L[5] | |||
| Solubility | Soluble in ethanol, diethyl ether, benzene, toluene, methanol, acetone and insoluble in water | ||
| log P | 1.09 | ||
Henry's law
constant (kH) |
14 nmol/(Pa·kg) | ||
| Conjugate acid | Methanium | ||
| Conjugate base | Methyl anion | ||
| −17.4×10−6 cm3/mol[6] | |||
| Structure | |||
| Td | |||
| Tetrahedral at carbon atom | |||
| 0 D | |||
| Thermochemistry[7] | |||
Heat capacity (C)
|
35.7 J/(K·mol) | ||
Std molar
entropy (S⦵298) |
186.3 J/(K·mol) | ||
Std enthalpy of
formation (ΔfH⦵298) |
−74.6 kJ/mol | ||
Gibbs free energy (ΔfG⦵)
|
−50.5 kJ/mol | ||
Std enthalpy of
combustion (ΔcH⦵298) |
−891 kJ/mol | ||
| Hazards[8] | |||
| GHS labelling: | |||

| |||
| Danger | |||
| H220 | |||
| P210 | |||
| NFPA 704 (fire diamond) | |||
| Flash point | −188 °C (−306.4 °F; 85.1 K) | ||
| 537 °C (999 °F; 810 K) | |||
| Explosive limits | 4.4–17% | ||
| Related compounds | |||
Related alkanes
|
|||
Related compounds
|
|||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
{{Chembox
| Verifiedfields = changed
| Watchedfields = changed
| verifiedrevid = 464371375
| Name =
| ImageFile = Methane-2D-dimensions.svg
| ImageFile_Ref = {{chemboximage|correct|??}}
| ImageSize = 170
| ImageName = Stereo, skeletal formula of methane with some measurements added
| ImageFileL1 = Methane-CRC-MW-3D-balls.png
| ImageFileL1_Ref = {{chemboximage|correct|??}}
| ImageNameL1 = Ball and stick model of methane
| ImageFileR1 = Methane-3D-space-filling.svg
| ImageFileR1_Ref = {{chemboximage|correct|??}}
| ImageNameR1 = Spacefill model of methane
| ImageCaptionR1 = {{legend|black|Carbon, C}}{{legend|white|Hydrogen, H}}
| PIN = Methane<ref name=iupac2013>{{cite book|title = Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book)|publisher = [[Royal Society of Chemistry|The Royal Society of Chemistry]]|date = 2014|location = Cambridge|pages = 3–4|doi = 10.1039/9781849733069-FP001|isbn = 978-0-85404-182-4|quote = Methane is a retained name (see P-12.3) that is preferred to the systematic name ‘carbane’, a name never recommended to replace methane, but used to derive the names ‘carbene’ and ‘carbyne’ for the radicals H<sub>2</sub>C<sup>2•</sup> and HC<sup>3•</sup>, respectively.|chapter = Front Matter}}</ref>
| SystematicName = Carbane (never recommended<ref name=iupac2013 />)
| OtherNames = {{ubl|Carbon tetrahydride|Carburetted hydrogen|Hydrogen carbide|Marsh gas|Methyl hydride|Natural gas}}
| IUPACName =
| Section1 = {{Chembox Identifiers
| CASNo = 74-82-8
| CASNo_Ref = {{cascite|correct|CAS}}
| UNII_Ref = {{fdacite|correct|FDA}}
| UNII = OP0UW79H66
| PubChem = 297
| ChemSpiderID = 291
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}}
| EINECS = 200-812-7
| UNNumber = 1971
| KEGG = C01438
| KEGG_Ref = {{keggcite|confirmed|kegg}}
| MeSHName = Methane
| ChEBI = 16183
| ChEBI_Ref = {{ebicite|correct|EBI}}
| ChEMBL = 17564
| ChEMBL_Ref = {{ebicite|correct|EBI}}
| RTECS = PA1490000
| Beilstein = 1718732
| Gmelin = 59
| 3DMet = B01453
| SMILES = C
| StdInChI = 1S/CH4/h1H4
| StdInChI_Ref = {{stdinchicite|correct|chemspider}}
| StdInChIKey = VNWKTOKETHGBQD-UHFFFAOYSA-N
| StdInChIKey_Ref = {{stdinchicite|correct|chemspider}}
}}
| Section2 = {{Chembox Properties
| C=1 | H=4
| Appearance = Colorless gas
| Odor = Odorless
| Density = {{ubl|0.657 kg/m<sup>3</sup> (gas, 25 °C, 1 atm)|0.717 kg/m<sup>3</sup> (gas, 0 °C, 1 atm)<ref>{{cite web|title=Gas Encyclopedia|url=http://encyclopedia.airliquide.com/Encyclopedia.asp?GasID=41|access-date=November 7, 2013|archive-date=December 26, 2018|archive-url=https://web.archive.org/web/20181226083050/https://encyclopedia.airliquide.com/methane?GasID=41|url-status=live}}</ref>
| 422.8 g/L (liquid, −162 °C)<ref name=crc>[[#Haynes|Haynes]], p. 3.344</ref>
}}
| MeltingPtC = −182.456
| BoilingPtC = −161.5
| MeltingPt_ref = <ref name=crc/>
| BoilingPt_ref = <ref name=crc/>
| CriticalTP = {{cvt|190.56|K}}, {{cvt|4.5992|MPa|atm}}
| Solubility = 22.7 mg/L<ref>[[#Haynes|Haynes]], p. 5.156</ref>
| SolubleOther = Soluble in [[ethanol]], [[diethyl ether]], [[benzene]], [[toluene]], [[methanol]], [[acetone]] and insoluble in [[water]]
| ConjugateAcid = [[Methanium]]
| ConjugateBase = [[Methyl anion]]
| LogP = 1.09
| HenryConstant = 14 nmol/(Pa·kg)
| MagSus = −17.4{{e|−6}} cm<sup>3</sup>/mol<ref>[[#Haynes|Haynes]], p. 3.578</ref>
}}
| Section3 = {{Chembox Structure
| MolShape = [[Tetrahedral molecular geometry|Tetrahedral]] at [[carbon]] atom
| Dipole = 0{{nbsp}}D
| PointGroup = T<sub>d</sub>
}}
| Section4 = {{Chembox Thermochemistry
| Thermochemistry_ref = <ref>[[#Haynes|Haynes]], pp. 5.26, 5.67</ref>
| DeltaGfree = −50.5 kJ/mol
| DeltaHf = −74.6 kJ/mol
| DeltaHc = −891 kJ/mol
| Entropy = 186.3 J/(K·mol)
| HeatCapacity = 35.7 J/(K·mol)
}}
| Section7 = {{Chembox Hazards
| Hazards_ref = <ref>{{cite web|title=Safety Datasheet, Material Name: Methane|url=http://www.chemadvisor.com/Matheson/database/msds/00244226000800003.PDF|publisher=Metheson Tri-Gas Incorporated|access-date=December 4, 2011|location=US|date=December 4, 2009|url-status=dead|archive-url=https://web.archive.org/web/20120604162221/http://www.chemadvisor.com/Matheson/database/msds/00244226000800003.PDF|archive-date=June 4, 2012}}</ref>
| GHSPictograms = {{GHS02}}
| GHSSignalWord = Danger
| HPhrases = {{H-phrases|220}}
| PPhrases = {{P-phrases|210}}
| NFPA-H = 2
| NFPA-F = 4
| NFPA-R = 0
| NFPA-S = SA
| FlashPtC = −188
| AutoignitionPtC = 537
| ExploLimits = 4.4–17%
}}<ref>{{cite web|url=http://cameochemicals.noaa.gov/chemical/8823|title=METHANE|author=NOAA Office of Response and Restoration, US GOV|website=noaa.gov|access-date=March 20, 2015|archive-date=January 9, 2019|archive-url=https://web.archive.org/web/20190109075841/https://cameochemicals.noaa.gov/chemical/8823|url-status=live}}</ref>
| Section8 = {{Chembox Related
| OtherFunction_label = alkanes
| OtherFunction = {{ubl|[[Ethane]]|[[Propane]]|[[Butane]]}}
| OtherCompounds = {{ubl|[[Silane]]|[[Germane]]|[[Stannane]]|[[Plumbane]]}}
}}
}}
Here is the example for human hemoglobin, a protein, on the right hand side is the final result, beneath is the wikicode.
| hemoglobin | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (heterotetramer, (αβ)2) | |||||||||||||
 Structure of human hemoglobin. α and β subunits are in red and blue, and the iron-containing heme groups in green. From PDB: 1GZX Proteopedia Hemoglobin | |||||||||||||
| Protein type | metalloprotein, globulin | ||||||||||||
| Function | oxygen-transport | ||||||||||||
| Cofactor(s) | heme (4) | ||||||||||||
| |||||||||||||
{{Infobox heteropolypeptide
| heteropolymer = hemoglobin
| polymer_type = heterotetramer, (αβ)<sub>2</sub>
| protein_type = [[metalloprotein]], [[globulin]]
| function = [[oxygen]]-transport
| cofactors = [[heme]] (4)
| image = 1GZX Haemoglobin.png
| image_source = Structure of human hemoglobin. <span style="color:red;">'''α'''</span> and <span style="color:blue;">'''β'''</span> subunits are in red and blue, and the iron-containing [[heme]] groups in green. From {{PDB|1GZX}} {{Proteopedia|Hemoglobin}}
| SubunitCount = 3
| subunit1 = Hb-α1
| gene1 = [[HBA1]]
| locus1 = [[Chromosome 16|Chr. 16]] [https://www.ncbi.nlm.nih.gov/Omim/getmap.cgi?chromosome=16p13.3 p13.3]
| subunit2 = Hb-α2
| gene2 = [[HBA2]]
| locus2 = [[Chromosome 16|Chr. 16]] [https://www.ncbi.nlm.nih.gov/Omim/getmap.cgi?chromosome=16p13.3 p13.3]
| subunit3 = Hb-β
| gene3 = [[HBB]]
| locus3 = [[Chromosome 11|Chr. 11]] [https://www.ncbi.nlm.nih.gov/Omim/getmap.cgi?chromosome=11p15.5 p15.5]
|Formula=C2952 H 4664 O 832 N812 S8 Fe 4|C2952 H 4664 O 832 N812 S8 Fe 4=}}
Here is the example for King-Wai Yau, a scientist: final result on the right, wikicode beneath this sentence.
King-Wai Yau | |
|---|---|
 King-Wai Yau delivering 2008 Champalimaud Award lecture at ARVO meeting | |
| Born | October 27, 1948 Guangzhou (Canton), China |
| Citizenship | USA |
| Alma mater | Princeton A.B. in physics 1971, Harvard Ph.D in neurobiology 1975 |
| Known for | mechanisms of sensory transduction in vision and olfaction |
| Scientific career | |
| Fields | Neuroscience, Biophysics |
| Institutions | Johns Hopkins University, University of Texas Medical Branch |
| Academic advisors | John G. Nicholls, Denis A. Baylor, Alan L. Hodgkin |
| Website | neuroscience |
{{Infobox scientist
| name = King-Wai Yau
| image = King-Way Yau 2008 ARVO.jpg
| alt = photo of King-Wai Yau
| caption = King-Wai Yau delivering 2008 Champalimaud Award lecture at ARVO meeting
| birth_date = {{Birth date and age|1948|10|27}}
| birth_place = Guangzhou (Canton), China
| citizenship = USA
| nationality =
| fields = [[Neuroscience]], [[Biophysics]]
| workplaces = [[Johns Hopkins University]], [[University of Texas Medical Branch]]
| alma_mater = [[Princeton University|Princeton]] A.B. in physics 1971, [[Harvard]] Ph.D in neurobiology 1975
| known_for = mechanisms of sensory transduction in vision and olfaction
| academic_advisors= John G. Nicholls, Denis A. Baylor, [[Alan Lloyd Hodgkin|Alan L. Hodgkin]]
| website = {{URL|neuroscience.jhu.edu/KingWaiYau.php}}
}}
List of science-related infoboxes
[edit]For biology :
Wikipedia:List_of_infoboxes#Biology
For other topics:
Wikipedia:List_of_infoboxes#Other_science_and_nature
How to cite references
[edit]An assertion needs to be backed with a citation. To introduce it in a Wikipedia article, you can rely on the visual editor for most cases (books, websites, articles). In certain cases, you will need to know the wikicode (for citing a thesis, for example). Below is an example of wikicode concerning an article, and further below, the links to the pages explaining how to cite books, websites, and thesis.
Journal articles
[edit]To achieve the following note,[10] you can both rely on the visual editor, or use the following wikicode:
<ref>
{{cite journal
|last = Peyron
|first = Marie-Agnès
|author2 = Olivier Blanc
|author3 = James P. Lund
|author4 = Alain Woda
|date = 2004-03-09
|title = Influence of Age on Adaptability of Human Mastication
|journal = [[Journal of Neurophysiology]]
|volume = 92
|issue = 2
|pages = 773–779
|url = http://jn.physiology.org/cgi/content/full/92/2/773
|doi = 10.1152/jn.01122.2003
|pmid = 15277595
}}
</ref>
Useful external tool:
How to cite books, webpages, or audiovisual media
[edit]Audiovisual media template here
Different wikiprojects that the articles you create might belong to
[edit]Biophysics Project
[edit]Please post this on the talk pages of biophysics-related articles.
| Biophysics (inactive) | ||||
| ||||
Feel free to place this on your user page after joining the project.
{{User WikiProject Biophysics}}
| This user participates in WikiProject Biophysics. |
Other projects
[edit]- Wikipedia_talk:WikiProject_Materials
- Wikipedia:WikiProject_Organismal_Biomechanics
- Wikipedia:WikiProject_Molecular_and_Cell_Biology
- Wikipedia:WikiProject_Chemical_and_Bio_Engineering
- Wikipedia:WikiProject_Fungi
For a full list, see here.
Supporting editors
[edit]Students: To contact a helper, here is the link to their talk page:
Supporting pages
[edit]Topic suggestions:
- Compounds or compound classes that don't have an article on English Wikipedia: https://w.wiki/AJYN
- Metabolism of proteins
- Chemists who don't have an article on English Wikipedia: https://w.wiki/AJrG
- German chemists who don't have an article on English Wikipedia: https://w.wiki/AJrP
- Indian chemists who don't have an article on English Wikipedia: https://w.wiki/AJrR
- Dutch chemists who don't have an article on English Wikipedia: https://w.wiki/AJrV
- Creating set index pages like: C18H36O2
Suggested sources of media related to Chemistry:

 Results of the workshop
Results of the workshop
[edit](please add a link to your editing work)
![]() Wikipedia articles (new, expanded, improved):
Wikipedia articles (new, expanded, improved):
- Artificial metalloenzyme (article improved)
- Stratingh Institute for Chemistry (article improved, Wikidata added)
- Double-stranded RNA (create new article from subsection of RNA)
- Horseradish peroxidase и Пероксидаза хрена (new subsection for this article created)
- Methylester (new article created)
- QM/MM(improve article)
- Semi rational design (new section to existing article Protein design created)
- Metallopeptide (new article created)
- Cyclodextrin (existing article expanded)
- Dxcover (new article created)
- AXL receptor tyrosine kinase (improved article)
- Enzymatic polymerization (new article created)
- Scale-down bioreactor (new article created)
- Photo-ATRP (Photoinduced Atom Transfer Radical Polymerization) (new article created)
- OrthoRep (new article created)
- Escherichia coli BL21(DE3) (new article created)
- Peptide_synthesis (added figure illustrating the synthesis steps)
- Biometal_(biology) (added subsection "Non-natural biometal complexes" and updated page generally)
 Participants
Participants
[edit]To insert your individual username, please choose "Edit source" and replace your name here by this string: *~~~~
- Basman0va (talk)
- Biopaprika (talk)
- CemToyKök (talk)
- Hemadzer (talk)
- Joenikesh (talk)
- King of the north Snow (talk)
- Lena Willhoeft (talk)
- MONOX1234 (talk)
- Mslanska (talk)
- OceanWaves (talk)
- PeterFrasko (talk)
- Qianxin.chemist (talk)
- Vara104 (talk)
Further reading
[edit]- Wikipedia Editing for Academicians - by Dr. Thomas Shafee
- Ten Simple Rules for Editing Wikipedia - A beginners guide published by PLOS Comp Biol
- Introduction to editing - a series of 5-10 minute tutorials
- Getting started - A collection of useful links for new editors
- some useful links in German
- Policy on the usage of Artificial Intelligence on English Wikipedia.
- Rapid Grants
- ...
References
[edit]- ^ "Developing novel catalysts from enzymes PhD network MetRaZymes receives funding as Marie Skłodowska-Curie action | News | Nov 28, 2022 | Institute of Biochemical Engineering | University of Stuttgart". Retrieved 2024-05-26.
- ^ a b "Front Matter". Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. pp. 3–4. doi:10.1039/9781849733069-FP001. ISBN 978-0-85404-182-4.
Methane is a retained name (see P-12.3) that is preferred to the systematic name 'carbane', a name never recommended to replace methane, but used to derive the names 'carbene' and 'carbyne' for the radicals H2C2• and HC3•, respectively.
- ^ "Gas Encyclopedia". Archived from the original on December 26, 2018. Retrieved November 7, 2013.
- ^ a b c Haynes, p. 3.344
- ^ Haynes, p. 5.156
- ^ Haynes, p. 3.578
- ^ Haynes, pp. 5.26, 5.67
- ^ "Safety Datasheet, Material Name: Methane" (PDF). US: Metheson Tri-Gas Incorporated. December 4, 2009. Archived from the original (PDF) on June 4, 2012. Retrieved December 4, 2011.
- ^ NOAA Office of Response and Restoration, US GOV. "METHANE". noaa.gov. Archived from the original on January 9, 2019. Retrieved March 20, 2015.
- ^ Peyron, Marie-Agnès; Olivier Blanc; James P. Lund; Alain Woda (2004-03-09). "Influence of Age on Adaptability of Human Mastication". Journal of Neurophysiology. 92 (2): 773–779. doi:10.1152/jn.01122.2003. PMID 15277595. Retrieved 2008-07-02.



