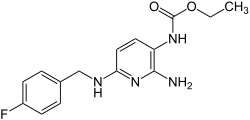
Flupirtine
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| ATC code | |
| Pharmacokinetic data | |
| Bioavailability | 90% (oral), 70% (rectal)[1] |
| Metabolism | Hepatic to 2-amino-3-acetylamino-6-(para-fluorobenzylamino) pyridine (which has 20-30% the analgesic potential of its parent compound), para-fluorohippuric acid[3] and a mercapturic acid metabolite, presumably formed from a glutathione adduct[4] |
| Elimination half-life | 6.5 hrs (average), 11.2-16.8 hrs (average 14 hrs) (elderly), 8.7-10.9 hrs (average 9.8 hrs) (in those with moderate-level renal impairment)[1] |
| Excretion | 72% of flupirtine and its metabolites appear in urine and 18% appear in feces[2] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.054.986 |
| Chemical and physical data | |
| Formula | C15H17FN4O2 |
| Molar mass | 304.325 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Flupirtine is an aminopyridine that functions as a centrally acting non-opioid analgesic that was originally used as an analgesic for acute and chronic pain[5] but in 2013 due to issues with liver toxicity, the European Medicines Agency restricted its use to acute pain, for no more than two weeks, and only for people who cannot use other painkillers.[6] In March 2018, marketing authorisations for flupirtine were withdrawn following a European Medicines Agency recommendation based on the finding that the restrictions introduced in 2013 had not been sufficiently followed in clinical practice, and cases of serious liver injury still occurred including liver failure.[7]
Flupirtine is a selective neuronal potassium channel opener (SNEPCO) that also has NMDA receptor antagonist and GABAA modulatory properties.[8]
It first became available in Europe in 1984 under the brand name Katadolon and after it went off patent many generic brands were introduced.[9]
Uses
[edit]Flupirtine is used as an analgesic for acute pain, in moderate-to-severe cases.[5][10] Its muscle relaxant properties make it popular for back pain and other orthopaedic uses, but it is also used for migraines, in oncology, postoperative care, and gynaecology.
In 2013 due to issues with liver toxicity, the European Medicines Agency restricted its use to acute pain, for no more than two weeks, and only for people who cannot use other painkillers.[6]
Side effects
[edit]The most serious side effect is frequent hepatotoxicity which prompted regulatory agencies to issue several warnings and restrictions.[11][12]
Flupirtine is devoid of negative psychological or motor function effects, or effects on reproductive function.[13][14]
Abuse and dependence
[edit]Although some studies have reported flupirtine has no addictive properties,[15][16] there was suggestion that it may possess some abuse potential and liability.[17] There were at least two registered cases of flupirtine abuse.[18] Drug tolerance does not develop in most cases, but has individually occurred.[18]
Mechanism of action
[edit]Flupirtine is a selective neuronal potassium channel opener that also has indirect NMDA receptor antagonist and GABAA receptor modulatory properties.[8][5]
History
[edit]Flupirtine was discovered and developed between the 1970s and the 1990s by Chemiewerk Homburg in Frankfurt am Main, Germany, which became Degussa Pharma Group and then through mergers, ASTA Pharma and Asta Medica.[8] Retigabine, in which the pyridine group in flupirtine is replaced with a phenyl group, was discovered as part of the same program and has a similar mechanism of action.[8]
It was approved for the treatment of pain in 1984 in Europe[19] under the brand name Katadolon.[20]
As of 2013 it was used in 11 member countries: Bulgaria, Estonia, Germany, Hungary, Italy, Latvia, Lithuania, Poland, Portugal, Romania and Slovak Republic.[19] Many generics entered the European market around 2011.[21]
It was never introduced to the United States market for any indication but in 2008, Adeona Pharmaceuticals, Inc. (now called Synthetic Biologics, Inc.) obtained an option to license issued and patent pending applications relating to flupirtine's use in the treatment of ophthalmic indications, particularly retinitis pigmentosa.[22]
In 2010 retigabine was approved by the FDA as an anticonvulsant for the treatment of refractory partial-onset seizures in treatment-experienced patients.[23]
As of 2016 it is marketed under many brand names, including Efiret, Flupigil, Flupirtin, Flupirtina, Flupirtine, Flupizen, Fluproxy, Katadolon, Metanor, Trancolong, and Zentiva.[9]
Research
[edit]Flupirtine has been noted for its neuroprotective properties, and has been investigated for possible use in Creutzfeldt–Jakob disease, Alzheimer's disease, and multiple sclerosis.[24][25] It has also been proposed as a possible treatment for Batten disease.[26]
Flupirtine underwent a clinical trial as a treatment for multiple sclerosis[27] and fibromyalgia.[28] Flupirtine showed promise for fibromyalgia due to its different action than the three approved by U.S. FDA drugs: pregabalin, milnacipran, and duloxetine.[29] Additionally, there are case reports regarding flupirtine as a treatment for fibromyalgia.[30] Adeona Pharmaceuticals (now called Synthetic Biologics) sub-licensed its patents for using flupirtine for fibromyalgia to Meda AB in May 2010.[29]
References
[edit]- ^ a b Abrams SM, Baker LR, Crome P, White AS, Johnston A, Ankier SI, et al. (May 1988). "Pharmacokinetics of flupirtine in elderly volunteers and in patients with moderate renal impairment". Postgraduate Medical Journal. 64 (751): 361–363. doi:10.1136/pgmj.64.751.361. PMC 2428663. PMID 3200777.
- ^ Blackburn-Munro G, Dalby-Brown W, Mirza NR, Mikkelsen JD, Blackburn-Munro RE (2005). "Retigabine: chemical synthesis to clinical application". CNS Drug Reviews. 11 (1): 1–20. doi:10.1111/j.1527-3458.2005.tb00033.x. PMC 6741764. PMID 15867950.
- ^ Narang PK, Tourville JF, Chatterji DC, Gallelli JF (January 1984). "Quantitation of flupirtine and its active acetylated metabolite by reversed-phase high-performance liquid chromatography using fluorometric detection". Journal of Chromatography. 305 (1): 135–143. doi:10.1016/S0378-4347(00)83321-6. PMID 6707137.
- ^ Methling K, Reszka P, Lalk M, Vrana O, Scheuch E, Siegmund W, et al. (March 2009). "Investigation of the in vitro metabolism of the analgesic flupirtine". Drug Metabolism and Disposition. 37 (3): 479–493. doi:10.1124/dmd.108.024364. PMID 19074524. S2CID 5661841.
- ^ a b c Harish S, Bhuvana K, Bengalorkar GM, Kumar T (April 2012). "Flupirtine: Clinical pharmacology". Journal of Anaesthesiology Clinical Pharmacology. 28 (2): 172–177. doi:10.4103/0970-9185.94833. PMC 3339720. PMID 22557738.
- ^ a b "Flupirtine-containing medicines". European Medicines Agency. November 21, 2013.
- ^ "European Medicines Agency - Human medicines - Flupirtine-containing medicinal products". www.ema.europa.eu. Archived from the original on 2022-01-21. Retrieved 2018-03-18.
- ^ a b c d Szelenyi I (March 2013). "Flupirtine, a re-discovered drug, revisited". Inflammation Research. 62 (3): 251–258. doi:10.1007/s00011-013-0592-5. PMID 23322112. S2CID 16535456.
- ^ a b Flupirtine Drugs.com. Accessed 30 August 2016
- ^ McMahon FG, Arndt WF, Newton JJ, Montgomery PA, Perhach JL (1987). "Clinical experience with flupirtine in the U.S". Postgraduate Medical Journal. 63 Suppl 3 (3): 81–85. PMID 3328854.
- ^ EMA information about flupirtine
- ^ "Flupirtin: EMA startet Risikobewertung wegen Leberrisiko". Deutsches Ärzteblatt. 15 March 2013.
- ^ Singal R, Gupta P, Jain N, Gupta S (June 2012). "Role of flupirtine in the treatment of pain - chemistry and its effects" (PDF). Maedica. 7 (2): 163–166. PMC 3557425. PMID 23401726.
- ^ "DRUGDEX Evaluations - Flupirtine". Retrieved 24 March 2013.
- ^ Preston KL, Funderburk FR, Liebson IA, Bigelow GE (March 1991). "Evaluation of the abuse potential of the novel analgesic flupirtine maleate". Drug and Alcohol Dependence. 27 (2): 101–113. doi:10.1016/0376-8716(91)90027-v. PMID 2055157.
- ^ Sofia RD, Diamantis W, Gordon R (1987). "Abuse potential and physical dependence liability studies with flupirtine maleate in laboratory animals". Postgraduate Medical Journal. 63 (Suppl 3): 35–40. PMID 3447127.
- ^ Gahr M, Freudenmann RW, Connemann BJ, Hiemke C, Schönfeldt-Lecuona C (December 2013). "Abuse liability of flupirtine revisited: implications of spontaneous reports of adverse drug reactions". Journal of Clinical Pharmacology. 53 (12): 1328–1333. doi:10.1002/jcph.164. PMID 24037995. S2CID 35299692.
- ^ a b Stoessel C, Heberlein A, Hillemacher T, Bleich S, Kornhuber J (August 2010). "Positive reinforcing effects of flupirtine--two case reports". Progress in Neuro-Psychopharmacology & Biological Psychiatry. 34 (6): 1120–1121. doi:10.1016/j.pnpbp.2010.03.031. PMID 20362025. S2CID 19710997.
- ^ a b "Assessment report for flupirtine containing medicinal products" (PDF). EMA. June 24, 2013.
- ^ Allen RC (1986). "To Market, To Market - 1985". In Hesp B (ed.). Annual Reports in Medicinal Chemistry, Volume 21. Orlando: Academic Press. p. 328. ISBN 9780080583655.
- ^ "Rationale for the triggering of procedure under Article 107i of Directive 2001/83/EC on flupirtine presented by the Federal Institute for Drugs and Medicinal Devices/BfArM, Germany" (PDF). EMA. March 8, 2013.
- ^ "Adeona Pharmaceuticals and National Neurovision Research Institute (NNRI) Collaborate to Test Flupirtine for Retinitis Pigmentosa". Ann Arbor, MI and Owings Mills, MD: Synthetic Biologics, Inc. December 2, 2008. Archived from the original on 6 June 2014. Retrieved 2 June 2014.
- ^ "POTIGA (ezogabine) Tablets, CV. Full Prescribing Information Revised: September, 2013. Initial U.S. Approval: 2011" (PDF). GlaxoSmithKline and Valeant Pharmaceuticals. Retrieved 2 June 2014.
- ^ Klawe C, Maschke M (June 2009). "Flupirtine: pharmacology and clinical applications of a nonopioid analgesic and potentially neuroprotective compound". Expert Opinion on Pharmacotherapy. 10 (9): 1495–1500. doi:10.1517/14656560902988528. PMID 19505216. S2CID 11597721.
- ^ Swedberg MD, Shannon HE, Nickel B, Goldberg SR (September 1988). "Pharmacological mechanisms of action of flupirtine: a novel, centrally acting, nonopioid analgesic evaluated by its discriminative effects in the rat". The Journal of Pharmacology and Experimental Therapeutics. 246 (3): 1067–1074. PMID 2901483.
- ^ Dhar S, Bitting RL, Rylova SN, Jansen PJ, Lockhart E, Koeberl DD, et al. (April 2002). "Flupirtine blocks apoptosis in batten patient lymphoblasts and in human postmitotic CLN3- and CLN2-deficient neurons". Annals of Neurology. 51 (4): 448–466. doi:10.1002/ana.10143. PMID 11921051. S2CID 23653281.
- ^ Flupirtine as Oral Treatment in Multiple Sclerosis (FLORIMS) Clinical Trials.gov Accessed 20 September 2011.
- ^ Pipex Pharmaceuticals (PPXP)' Oral Flupirtine Receives IND With FDA for Phase II Clinical Trial for Fibromyalgia Archived 2017-08-30 at the Wayback Machine 4/21/2008
- ^ a b "Partnered Program. Effirma for Fibromyalgia". Synthetic Biologics, Inc. Archived from the original on 6 June 2014. Retrieved 2 June 2014.
- ^ Stoll AL, Belmont MA. (2000) "Fibromyalgia Symptoms Relieved by Flupirtine: An Open-Label Case Series Archived 2010-09-02 at the Wayback Machine" Psychosomatics 41:371-372. Accessed 20 September 2011.
