Molybdenum(II) acetate
| |
| Names | |
|---|---|
| Other names
Dimolybdenum tetraacetate,
tetra(aceto) dimolybdenum, Molybdenum(II) acetate dimer | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.034.611 |
| EC Number |
|
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C8H12Mo2O8 | |
| Molar mass | 428.1010 g/mol |
| Appearance | Yellow solids |
| Boiling point | decomposes |
| not soluble | |
| Hazards | |
| GHS labelling:[1] | |

| |
| Warning | |
| H315, H319 | |
| P222, P231, P235, P305+P351+P338, P422, P501 | |
| Safety data sheet (SDS) | External MSDS |
| Related compounds | |
Related compounds
|
Copper(II) acetate Chromium(II) acetate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
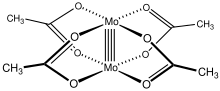
Molybdenum(II) acetate is a coordination compound with the formula Mo2(O2CCH3)4. It is a yellow, diamagnetic, air-stable solid that is slightly soluble in organic solvents. Molybdenum(II) acetate is an iconic example of a compound with a metal-metal quadruple bond.[2]
Structure
[edit]Like several other transition metal carboxylate complexes, Mo2(O2CCH3)4 adopts a Chinese lantern structure.[3] Each Mo(II) center in Mo2(O2CCH3)4 has four d valence electrons. These eight d-electrons form one σ, two π bonds, and one δ bond, creating a bonding electron configuration of σ2π4δ2. Each of these bonds are formed by the overlapping of pairs of d orbitals.[4] The four acetate groups bridge the two metal centers. The Mo-O bond between each Mo(II) center and O atom from acetate has a distance of 2.119 Å, and the Mo-Mo distance between the two metal centers is 2.0934 Å.
Preparation
[edit]Mo2(O2CCH3)4 is prepared by treating molybdenum hexacarbonyl (Mo(CO)6) with acetic acid. The process strips CO ligands from the hexacarbonyl and results in the oxidation of Mo(0) to Mo(II).[5][6]
- 2 Mo(CO)6 + 4 HO2CCH3 → Mo2(O2CCH3)4 + 12 CO + 2 H2
Trinuclear clusters are byproducts.[7]
The reaction of HO2CCH3 and Mo(CO)6 was first investigated by Bannister et al. in 1960. At the time, quadruple metal-metal bonds had not yet been discovered, so these authors proposed that Mo(O2CCH3)2 was tetrahedral.[8][9] This perspective changed with Mason's characterization.[10]
Applications
[edit]Mo2(O2CCH3)4 is generally used as an intermediate compound in a process to form other quadruply bonded molybdenum compounds.[2] The acetate ligands can be replaced to give new compounds such as [Mo2Cl8]4− and Mo2Cl4[P(C4H9)3]4.[2][11][12]
References
[edit]- ^ "Molybdenum (CAS Number 14221-06-8) : Strem Product Catalog". www.strem.com. Retrieved 27 December 2021.
- ^ a b c Girolami, G. S.; Rauchfuss, T. B. and Angelici, R. J., "Synthesis and Technique in Inorganic Chemistry third edition", University Science Books: Mill Valley, CA, 1999, ISBN 0-935702-48-2
- ^ Cotton, F. A.; Hillard, E.A.; Murillo, C. A.; Zhou, H.-C. "After 155 Years, A Crystalline Chromium Carboxylate with a Supershort Cr-Cr Bond" J. Am. Chem. Soc., 2000, 122, 416-417. doi:10.1021/ja993755i.
- ^ Blaudeau, J. P.; Pitzer, R. M. “ Ab Initio Studies of Ligand Effects on the Metal-Metal Bond in Dimolybdenum Complexes” J.Phys. Chem. 1994, vol. 98, pp. 4575-4579.
- ^ Brignole, A.G.; Cotton, F.A., “Rhenium and Molybdenum compounds containing quadruple compounds” Inorg. Synth. 1972, volume 13, pp. 81-89. doi:10.1002/9780470132449.ch15
- ^ Pence, L. E.; Weisgerber, A. M.; Maounis, F.A.; “Synthesis of Molybdenum-Molybdenum Quadruple Bonds” J. Chem. Educ., 1999, vol. 76, 404-405.
- ^ Bino, A.; Cotton, F.A.; Dori, A.; J. Am. Chem. Soc. 1981, vol. 103, pp. 243-244. “A Aqueous New Chemistry of Organometallic, Trinuclear Cluster Compounds of Molybdenum”.
- ^ Bannister, E.; Wikinson, G. “Molybdenum(II) carboxylates” Chem. Ind. 1960, 319.
- ^ Stephenson, T.A.; Bannister, E.; Wilkinson, G. “Molybdenum(II) Carboxylates” J. Chem. Soc., 1964, pp. 2538. doi:10.1039/JR9640002538
- ^ D. Lawton, R. Mason "The Molecular Structure of Molybdenum(II) Acetate"J. Am. Chem. Soc. 1965, vol 87, pp 921–922. doi:10.1021/ja01082a046
- ^ Tsai, Y.C.; Chen H.Z.; Chang, C.C.; Yu, J.K.; Lee, G.H.; Wang, Y.; Kuo, T.S. “Journey from Mo-Mo Quadruple Bonds to Quintuple Bonds” J. Am. Chem. Soc., 2009, 131, 12534-12535. doi:10.1021/ja905035f
- ^ Handa, M.; Mikuriya, M.; Kotera, T.; Yamada, K.; Nakso, T.; Matsumoto, H.; Kasuga, K. “Linear Chain Compounds of Molybdenum(II) Acetate Linked by Pyazine, 4,4’-Bipyridine, and 1,4- Diazabicyclo[2.2.2]octane” Bull. Chem. Soc. Jpn., 1995,68, 2567-2572.
